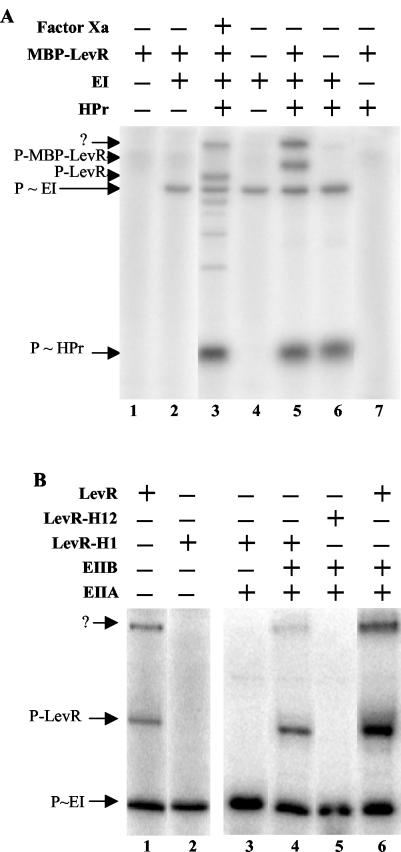
FIG. 6.
Phosphorylation of MBP-LevR by [32P]P∼His-HPr (A) and [32P]P∼EIIBLev (B). (A) Samples containing [32P]PEP and the indicated proteins were incubated at 37°C before they were separated on a 0.1% SDS-15% polyacrylamide gel which was dried and exposed to autoradiography. The sample loaded on lane 3 contained MBP-LevR, which had been treated with factor Xa before the phosphorylation reaction was carried out. The migration positions of HPr, EI, LevR, and MBP-LevR are indicated by arrows. The slowest-migrating radioactive band (arrow with the question mark) probably corresponds to MBP-LevR dimers. (B) Samples containing [32P]PEP, EI, HPr, and the indicated proteins were incubated at 37°C before they were separated on a 0.1% SDS-8% polyacrylamide gel, which was dried and exposed to autoradiography. In LevR-H1, His-488 in the EIIAMan domain was replaced with an alanine, while in LevR-H12, His-776 in PRD2 was also replaced with an alanine. The migration positions of EI and wild-type and mutant LevRs are indicated by arrows.