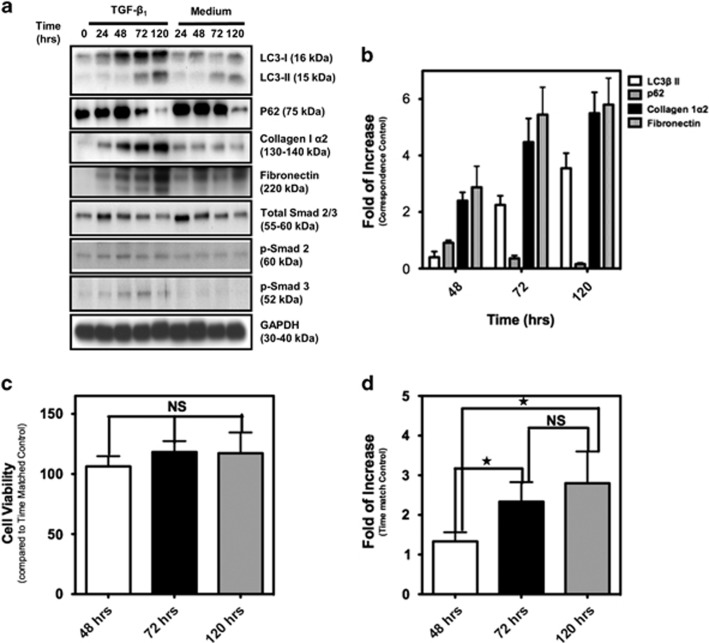
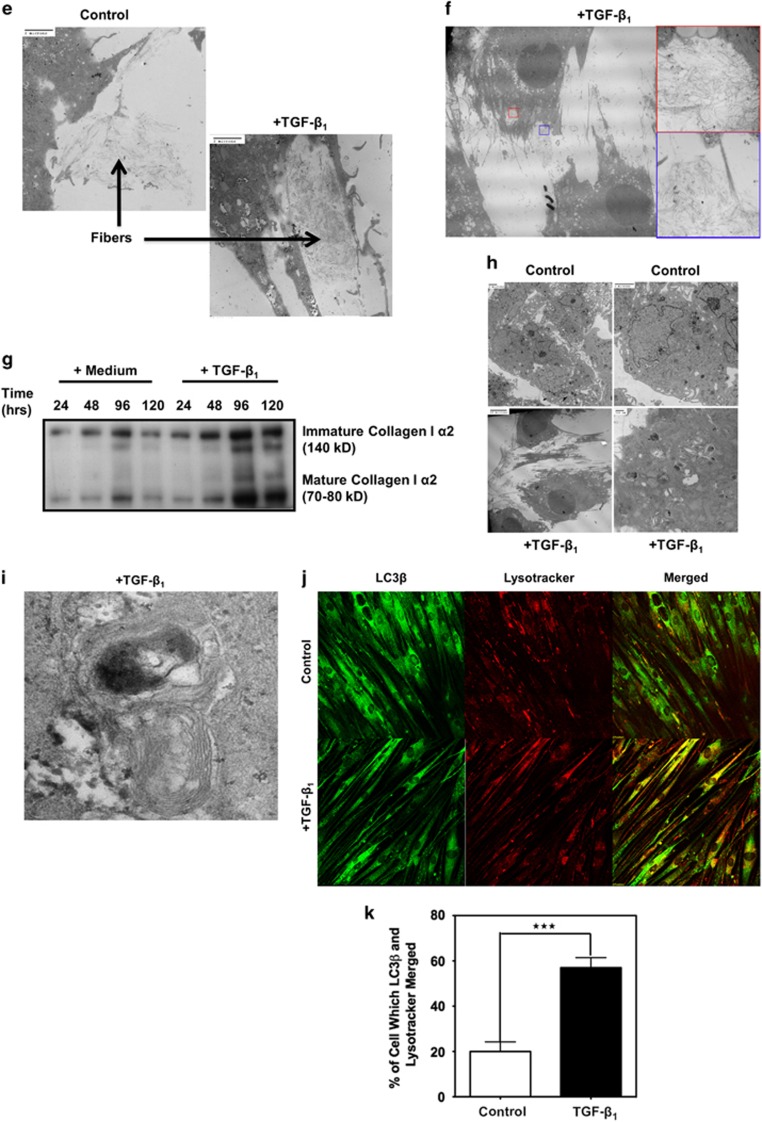
Figure 1.
TGFβ1 simultaneously induces fibrosis and autophagy in hATMyofbs. (a) Primary hATMyofbs (passages 2–5) were treated with TGF-β1 (10 ng/ml) for 0–120 h, and cell lysates were collected. Immunoblots were probed for the autophagy hallmark protein LC3β II, p62, as well as indicator proteins of the fibrogenic response in fibroblasts (i.e., collagen Iα2, fibronectin and the Smad signaling pathway). TGF-β1 induced LC3β II lipidation, with parallel increases in collagen Iα2, fibronectin protein expression and Smad2 and Smad3 phosphorylation. Data were normalized to GAPDH levels. Results are the means of three independent experiments from four different donors. (b) Densitometric analysis of LC3β II, p62, collagen Iα2 and fibronectin levels in hATMyofbs. Data are the means of three independent experiments from three different donors. For each experiment, LC3β II, collagen Iα2 and fibronectin levels were compared with those from time-matched controls and normalized to GAPDH levels. (c and d) TGF-β1 treatment does not affect cell viability of hATMyofbs, but it associated with their proliferation at 72 and 120 h. hATMyofbs were exposed to TGF-β1 (10 ng/ml) for the indicated time points (48, 72, 120 h), and cell viability and proliferation was measured as described in the Materials and Methods section in three different culture experiments (n=3). TGF-β1 treatment was not associated with any significant changes in cell viability (*P<0.01) (c) while it induced significant hATMyofb proliferation at 72 and 120 h compared with 48 h (P<0.01). (e and f) hATMyofbs were either untreated or treated with 10 ng/ml TGF-β1 for 96 h. Cells were then imaged by TEM at a magnification of 15 600 (e) and 6750 (f). Extracellular fiber deposition (collagen type I or fibronectin) was compared between the control and TGF-β1 treatment groups. TGF-β increased extracellular fiber deposition. (g) hATMyofbs were either untreated or treated with 10 ng/ml TGF-β1 for the indicated time points (0–120 h). The cell culture medium was collected and concentrated with filter tube (MESH 20 kDa). Collagen Iα2 was probed in concentrated cell culture media. TGF-β1 increased mature and immature collagen secretion at different time points. (h and i) hATMyofbs were either untreated or treated with 10 ng/ml TGF-β1 for 96 h. Cells were then imaged by TEM at a magnification of 3600 (control, top panel left), 7500 (control, top panel right) and for TGF-β1 treatment (right panel 2750, left panel 27 500 and (i) 127 000). An autophagosome is highlighted in panel (i). (j) hATMyofbs treated with TGF-β1 (10 ng/ml, 96 h) showed increased LysoTracker Red DND-99 staining (a marker of lysosomal activation) and an increase in punctuate staining for LC3β (green), a marker of autophagy, and LC3β lysosomal co-localization. (k) hATMyofbs were treated with TGF-β1 (10 ng/ml, 96 h) and were immunostained for LC3β (green) and lysosomes (red). Ten different fields (10 cells in each field) were randomly chosen in control and TGF-β1 treatment and were counted manually by an operator. The percentage of yellow cells (merged LC3 and lysosomes) were compared between control and TGF-β1 treatment. TGF-β1 significantly increased the percentage of yellow cells, which indicated LC3β II lysosomal co-localization (***P <0.001). NS, not significant