Figure 1.
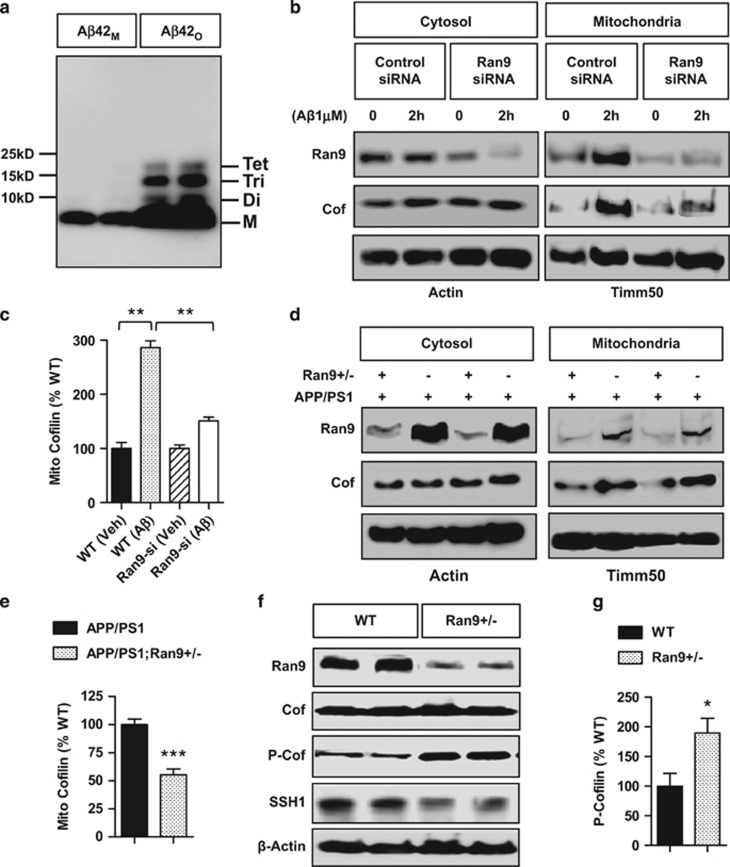
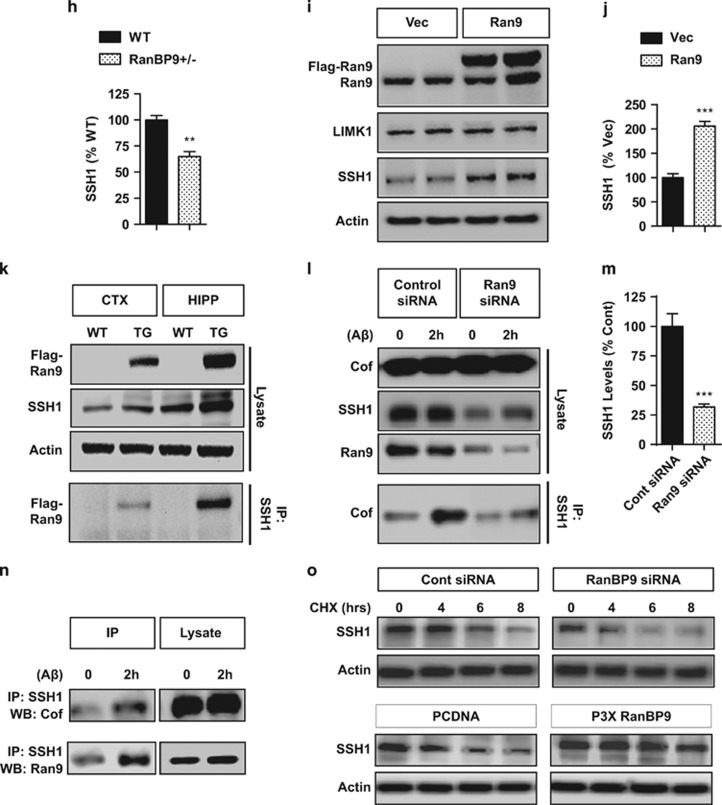
RanBP9 mediates AβO-induced translocation of cofilin to mitochondria and lowers cofilin activation via SSH1. (a) Freshly solubilized Aβ1-42 monomers (Aβ42M) or Aβ1-42 oligomer preparation (Aβ42O) subjected to SDS-PAGE and immunoblotted for Aβ. Note the monomer (M), dimers (Di), trimmers (Tri), and tetramemers (Tet) in the oligomer preparation. (b and c) Hippocampus-derived HT22 cells transiently transfected with control or RanBP9 siRNA for 48 h, treated with/without Aβ1-42O for 2 h, separated for mitochondrial and cytosol fractions, and subjected to immunoblotting for the indicated proteins. A representative experiment is shown. Notice the reduction phospho-cofilin upon Aβ42O treatment but mitigated response in RanBP9 siRNA knocked down cells. (c) Quantitation of mitochondrial cofilin (ANOVA, post-hoc Tukey, **P<0.005, n=3 replicates). (d and e) Hippocampal extracts from 3-month-old APP/PS1 and APP/PS1;RanBP9+/− mice subjected to separation of mitochondrial and cytosol fractions and immunoblotted for the indicated proteins. Note the reduced level of cofilin in mitochondrial fraction of APP/PS1;RanBP9+/− brain. (e) Quantitation of mitochondrial cofilin (t-test, **P<0.005, n=4 mice per genotype). (f-h) Hippocampal extracts from 3-month-old WT and littermate RanBP9+/− (Ran9+/−) mice subjected to immunoblotting for the indicated proteins. Representative experiment showing increase in phospho-cofilin (P-cofilin) and decrease in SSH1 in RanBP9+/− brain. (g) Quantitation of P-cofilin in hippocampus (t=2.74, P=0.022, *P<0.05, n=5 mice per genotype). (h) Quantitation of SSH1 (t-test, **P=0.0016, n=4 mice per genotype). (i and j) HT22 cells transiently transfected with vector control (Vec) or Flag-RanBP9 and immunoblotted for the indicated proteins. Note the increase in SSH1 but not LIMK1 by RanBP9 overexpression. (j) Quantitation of SSH1 (t-test, ***P<0.0001, n=4 replicates). Error bars represent S.E.M. on graphs. (k) Cortex (CTX) and hippocampus (HIPP) homogenates of 6-month-old WT or littermate Flag-RanBP9 transgenic (TG) mice immunoprecipitated for SSH1 and/or immunoblotted for the indicated proteins. Note the increase in SSH1-RanBP9 complex in Flag-RanBP9 transgenic mice (TG). (l) HT22 cells transiently transfected with/without RanBP9 siRNA and treated with/without Aβ42O (1 μM) for 2 h followed by immunoprecipitation for SSH1 and/or immunoblotting for the indicated proteins. Note that RanBP9 siRNA reduces SSH1 levels and decreases Aβ42O-induced enhancement of cofilin–SSH1 interaction. (m) Quantitation of SSH1 protein levels with/without RanBP9 siRNA transfection in HT22 cells (t-test, ***P=0.0008, n=4 replicates). (n) DIV18 cortical primary neurons treated with or without Aβ42O (1 μM) for 2 h, subjected to immunoprecipitation for SSH1, and/or immunoblotting for the indicated proteins. Note the increase in RanBP9-SSH1 and cofilin-SSH1 complex formation with Aβ42O treatment. (o) HT22 cells transiently transfected with control, RanBP9 siRNA, or RanBP9 and subjected to cycloheximide (CHX) treatment for the indicated times followed by immunoblotting for SSH1 or actin. Representative blots adjusted to show similar signal at time 0. Note that RanBP9 siRNA and overexpression accelerates and delays SSH1 turnover, respectively