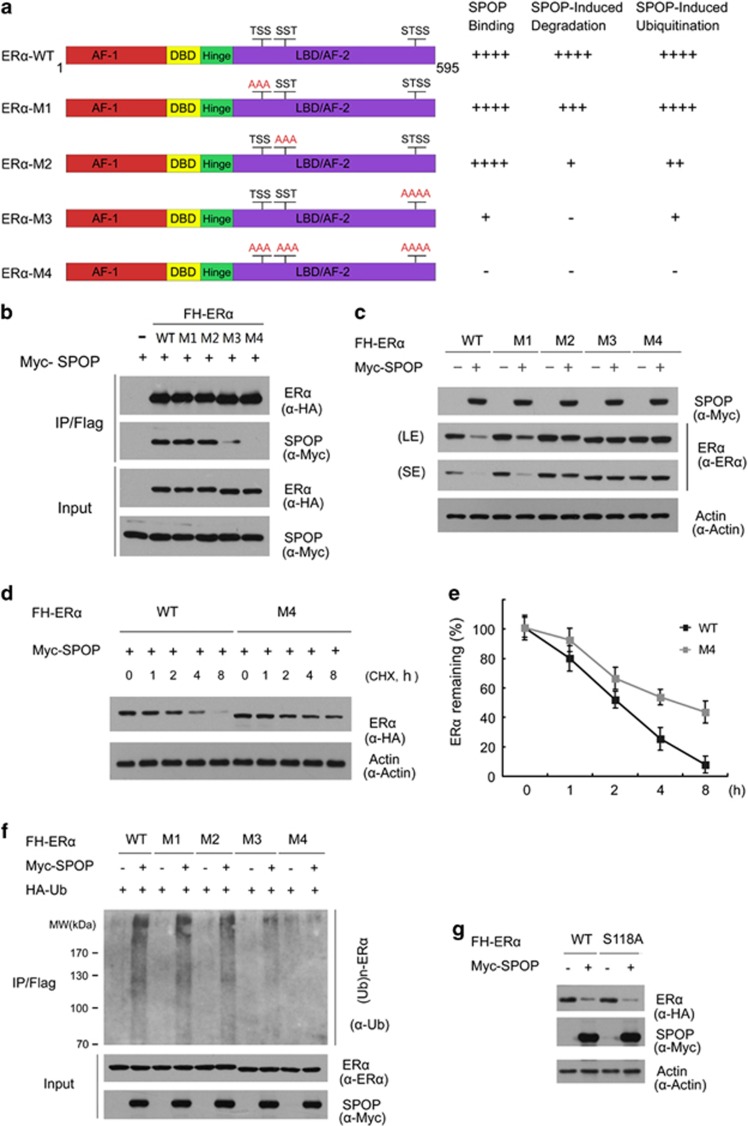
Figure 3.
The S/T-rich motifs in ERα are degrons recognized by SPOP. (a) Schematic representation of wild-type ERα protein with the upper contiguous Ser/Thr residues indicating the S/T-rich motifs in its amino-acid sequence. The ERα point mutants (M1, M2, M3, and M4) were constructed starting from the FH-ERα-WT vector are schematically reported below the wild-type protein. On the right of each schematic protein is summarized its SPOP-binding capacity, sensitivity to SPOP-induced degradation or ubiquitination. (b) The S/T-rich motifs in ERα are required for its binding to SPOP. The 293T cells were transfected with the indicated constructs. After 24 h, cell lysates were prepared for co-IP assay with anti-FLAG antibody and WB analyzes. (c) The S/T-rich motifs in ERα are required for SPOP-mediated ERα degradation. The 293T cells were transfected with the indicated constructs. After 24 h, cell lysates were prepared for WB analyzes. SE, short exposure; LE, long exposure. (d, e) Mutation of the S/T-rich motifs prolongs the half-life of ERα. ERα-WT or M4 mutant was transfected into 293T cells. After 24 h, cells were treated with 30 μM CHX. At the indicated time points, cell lysates were prepared for WB analyzes (d). At each time point, the intensity of ERα was first normalized to the intensity of Actin and then to the value of the 0-h time point (e). (f )The S/T-rich motifs are required for SPOP-mediated ERα polyubiquitination. The 293T cells were transfected with the indicated constructs. After 24 h, cells were treated with 20 μM MG132 for 4 h and the cell lysates were prepared for co-IP assay with anti-FLAG antibody and WB analyzes. The mean values (S.D.) of three independent experiments are shown. (g) Ser118 of ERα is not required for SPOP-mediated ERα degradation. The 293T cells were transfected with FH-ERα or S118A mutant in combination with or without Myc-SPOP constructs. After 24 h, cell lysates were prepared for WB analyzes