Fig. 1.
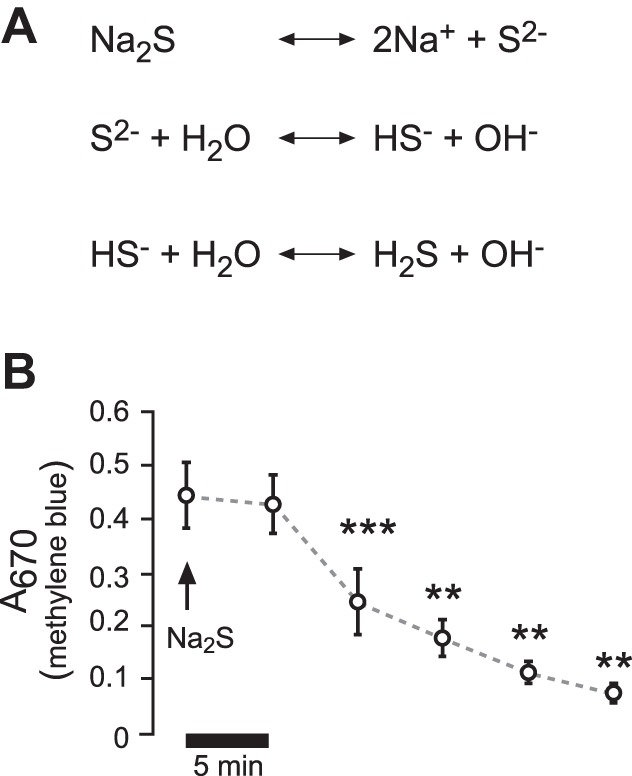
Administration of H2S and stability in buffer solutions. A: sulfur salt Na2S was employed to deliver H2S. According to the shown dissociation equations, a Na2S-containing solution would react basic. The employed buffers were adjusted, so that there were no pH changes with the employed Na2S concentrations (5 × 10−5 − 2.5 × 10−4 M). B: Na2S (5 × 10−5) was applied to buffers in Ussing chambers containing H441 Ringer solution, and aliquots were taken every 5 min. H2S was indirectly measured by the formation of methylene blue and its absorption at 670 nm. **P < 0.01, ***P < 0.001. Significant difference (Student's paired t-test) with respect to the initial value (n = 5).
