Figure 1.
Role of HECT E3 Ligases in Assembling Atypical Ub Chains
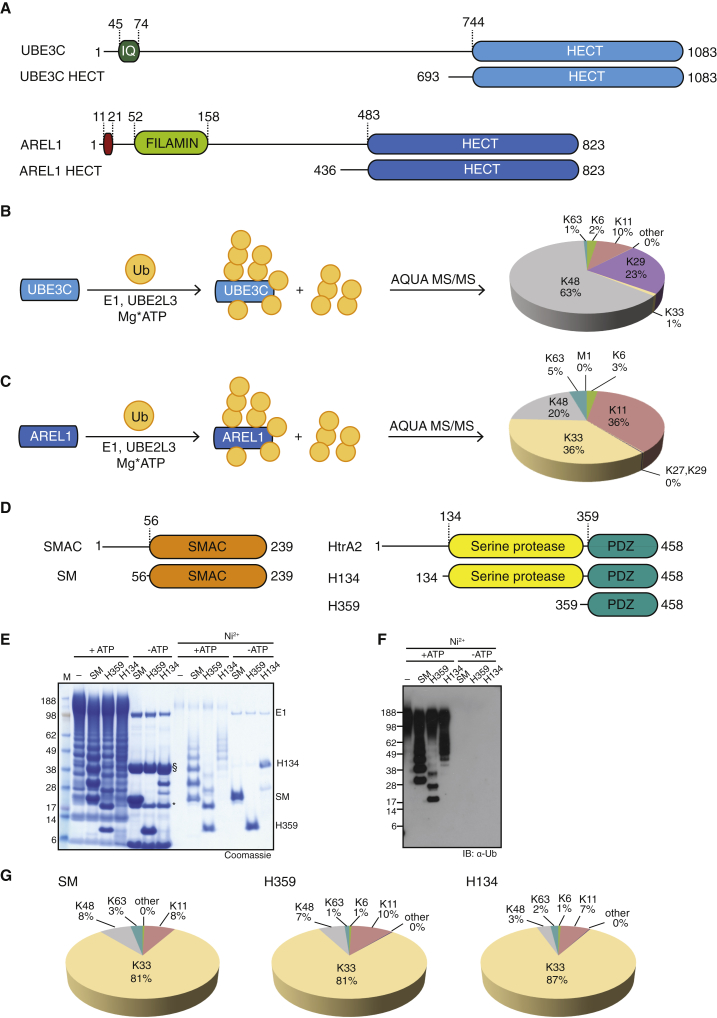
(A) Domain structures of UBE3C and AREL1 (KIAA0317) (top) and constructs used in this study (bottom).
(B) Schematic of an assembly reaction with UBE3C, UBE2L3 (UbcH7), E1, and WT Ub (left). The linkage composition in the reaction mixture was analyzed by AQUA-based MS/MS (right).
(C) Reaction as in (B) with AREL1, UBE2L3, E1, and WT Ub.
(D) Domain structures of the pro-apoptotic proteins SMAC and HtrA2 (top) and the expressed constructs used in this work (bottom).
(E) AREL1 is able to assemble chains onto SMAC and HtrA2 in an in vitro ubiquitination reaction that depends on ATP. Ubiquitinated, His6-tagged substrates are enriched following Ni2+ affinity binding. SM, SMAC (56–239); H359, HtrA2 (359–458); H134, HtrA2 (134–458); §, AREL1; ∗, UBE2L3.
(F) Western blot against Ub of the Ni2+-enriched reaction from (E).
(G) AQUA MS/MS profiles of the ubiquitinated substrates purified from (E).