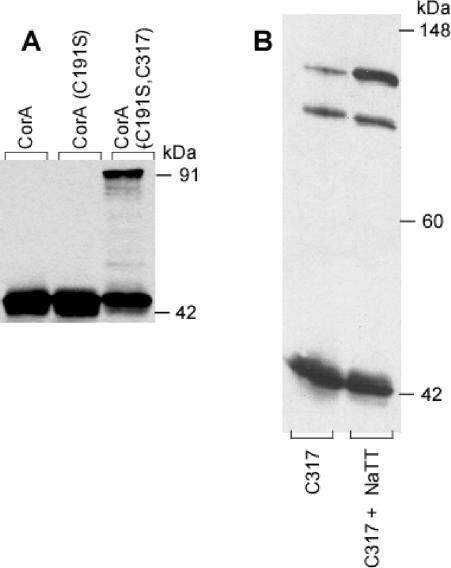
FIG. 1.
Addition of a Cys residue at the C terminus of CorA allows dimer formation. Salmonella serovar Typhimurium strains MM1318 (Cys191Ser), MM1322 (Cys317), and MM1324 (Cys191Ser Cys317) and a wild-type strain (MM1442) were grown in LB broth to an OD600 of 0.5 to 1.0, and membrane fractions were incubated with or without 10 mM tetrathionate. The samples were then run on nondenaturing PAGE before Western blotting with anti-CorA antibody and scanning into Canvas. No editing was performed except adjustment of brightness and contrast. Panel A shows the wild-type, the Cys− mutant, and the double mutant strains treated with tetrathionate. Some preparations showed a small amount of the 91-kDa band before tetrathionate oxidation (see panel B); the amount of the 91-kDa species was markedly increased by oxidation with tetrathionate. Panel B shows the formation of a third band at 110 kDa in the Cys 317 mutant containing both a cytoplasmic (Cys317) and a periplasmic (Cys191) cysteine. The band was enhanced by tetrathionate oxidation, in addition to the 91- and 44-kDa bands.