Abstract
BACKGROUND
Oxidized phospholipids on apolipoprotein B-100 (OxPL-apoB) is a biomarker of increased risk for major adverse cardiovascular events (MACE) in community cohorts, but its role in patients with stable coronary heart disease (CHD) is unknown.
OBJECTIVES
We sought to examine the relationship of these oxidative biomarkers to cardiovascular outcomes in patients with established CHD.
METHODS
In a random sample from the Treating to New Targets trial, OxPL-apoB levels were measured in 1,503 patients at randomization (after an 8-week run-in period on atorvastatin 10 mg) and 1 year after being randomized to atorvastatin 10 mg or 80 mg. We examined the association between baseline levels of OxPL-apoB and MACE, defined as death from CHD, nonfatal myocardial infarction (MI), resuscitation after cardiac arrest, and fatal/ nonfatal stroke, as well as the effect of statin therapy on OxPL-apoB levels and MACE.
RESULTS
Patients with events (n = 156) had higher randomization levels of OxPL-apoB than those without events (p = 0.025). For the overall cohort, randomization levels of OxPL-apoB predicted subsequent MACE (hazard ratio [HR]: 1.21; 95% confidence interval [CI]: 1.04 to 1.41; p = 0.018) per doubling and tertile 3 versus tertile 1 (HR: 1.69; CI: 1.14 to 2.49; p = 0.01) after multivariate adjustment for age, sex, body mass index (BMI), among others, and treatment assignment. The HR per doubling and by tertiles of OxPL-apoB remained significant in the low-dose but not the high-dose atorvastatin group.
CONCLUSIONS
Elevated OxPL-apoB levels predict secondary MACE in patients with stable CHD, a risk that is mitigated by atorvastatin 80 mg.
Keywords: atherosclerosis, biomarker, coronary heart disease, inflammatory, oxidation-specific epitope
Atherosclerosis is a chronic inflammatory disease whose pathogenesis is strongly mediated by dyslipidemia and other risk factors activating both innate and adaptive immune systems to generate chronic pro-inflammatory responses (1). Lipoprotein oxidation generates so-called “oxidation-specific epitopes” (OSE) that are an important, if not obligatory, intermediary between dyslipidemia, inflammation, and initiation and progression of atherosclerotic lesions. Clinical events ultimately result from the interaction of lipid accumulation, OSE generation, activation of inflammatory processes, recruitment of inflammatory cells to the vessel wall, endothelial dysfunction, platelet activation, and thrombosis.
These OSEs represent a major class of atherosclerosis-relevant antigens that define oxidative modifications on low-density lipoprotein cholesterol (LDL-C) particles, apoptotic cells, and proteins in the vessel wall (2). They are present both in the lipid phase as oxidized lipids and as oxidized lipids covalently bound to apolipoprotein B-100 (apoB) or other proteins. OSEs share molecular or immunological identity with danger-associated molecular patterns (known as DAMPs) on apoptotic cells and oxidized LDL-C, as well as pathogen-associated molecular patterns (or PAMPs) on microbial pathogens. Oxidation-specific epitopes are recognized by a common set of innate pattern recognition receptors, which have been evolutionary selected to protect against the pro-inflammatory properties of OSEs.
Phosphocholine (PC)-containing oxidized phospholipids (OxPL) are well studied OSEs that are highly immunogenic, pro-inflammatory, and present in atherosclerotic lesions of animals and humans, particularly in pathologically defined vulnerable and disrupted plaques (3). OxPLs are important contributors to early and late events in atherogenesis by activating pro-inflammatory genes in endothelial cells and macrophages (4), leading to inflammatory cascades in the vessel wall (5). Lipoprotein(a) [Lp(a)] is the major lipoprotein carrier of OxPL, which may impart pro-inflammatory properties (6,7). OxPL can be measured on apoB-100 lipoproteins (OxPL-apoB; reported as absolute units of phosphocholine-containing oxidized phospholipids [PC-OxPL] per unit apoB) in plasma with the murine natural antibody E06. OxPL-apoB levels have been shown to correlate with the presence of anatomical cardiovascular disease (CVD) (8) and events in clinical studies in community-based settings in patients generally without prior coronary heart disease (CHD) (9,10). The ability of OxPL-apoB to predict risk of angiographically-determined coronary artery disease (CAD) and CVD events is strongly potentiated by interleukin-1 genotypes that are associated with genetically-elevated predisposition to inflammation (11).
E06 binds to the PC headgroup of a variety of OxPL, but does not recognize phospholipids on unoxidized phospholipids or non-PC-oxidized phospholipids (12). Clinical outcome studies using alternative methods to measure OxPL, such as tandem liquid chromatography/mass spectrometry, have not been performed due to the laborious need to quantitate the multiple OxPL species and the lack of appropriate standards. Our group recently documented the presence of OSE in progressive coronary and carotid atheromata (3) and specific E06 and non-E06 detectable OxPL and oxidized cholesteryl esters by tandem liquid-chromatography/mass spectrometry in debris captured by distal protection devices in patients undergoing coronary or peripheral procedures, documenting their relevance to clinical disease (13,14).
The relationship of these oxidative biomarkers to cardiovascular outcomes in patients with established CHD is not defined. The aim of this study, therefore, was to assess the effect of low- versus high-dose statin therapy on OxPL-apoB levels in such patients and their relationship to subsequent major adverse cardiac events (MACE).
METHODS
STUDY DESIGN
This substudy is based on a random sample from the TNT (Treating to New Targets) study population, a randomized trial that compared the efficacy of high- (80 mg) versus low- (10 mg) dose atorvastatin for secondary prevention of CHD (15). Importantly for this substudy, blood samples were obtained at randomization, which occurred after an 8-week run-in period on atorvastatin 10 mg, and again 1 year after being randomized to 10 mg or 80 mg atorvastatin. This substudy consisted of 1,503 patients, of whom 1,347 did not experience an event and 156 had a documented MACE during the 4.9 years of study follow-up. MACE was defined as CVD death, nonfatal/ nonprocedure-related MI, resuscitated cardiac arrest, and fatal or nonfatal stroke.
OxPL-apoB levels were measured in a chemiluminescent immunoassay using the murine monoclonal antibody E06 that recognizes the PC group on oxidized but not on native phospholipids (Taleb et al. [8] and references therein). In prior studies, this variable was expressed as OxPL/apoB, reflecting the fact that this measure quantitates the number of OxPL moles per unit mass of apolipoprotein B-100 present on microtiter well plates (and not the level in the circulation). The nomenclature is now changed to OxPL-apoB to minimize confusion that this measure represents a ratio of OxPL divided by plasma levels of apoB. Details of the OxPL-apoB assay are provided in the Online Appendix. The Lp(a) levels were measured by a latex immonoturbidimetric assay (Wako Chemicals USA, Inc., Richmond, Virginia) (16).
STATISTICAL ANALYSIS
Patient demographics and disease characteristics at randomization were compared between treatment groups using a chi-square test for categorical variables and a Wilcoxon rank sum test for continuous variables. Similar comparisons were conducted between those who did and those who did not experience an event. Results in OxPL-apoB were also confirmed in a Student t test after a log transformation. The distribution of OxPL-apoB was highly skewed, but the normality was greatly improved after the log transformation. Changes from randomization (i.e., after 8 weeks of open-label run-in treatment with atorvastatin 10 mg) to year 1 in OxPL-apoB were tested with a Wilcoxon signed rank test, and compared with a Wilcoxon rank sum test between treatment groups and between patients with and without a clinical outcome. Spearman correlation was calculated to assess association between randomization and year 1 OxPL-apoB as well as between OxPL-apoB and lipid and other nonlipid biomarkers. The association between on-treatment OxPL-apoB (at time of randomization and at 1 year) and a MACE endpoint were assessed in a Cox proportional hazards model after adjustment for age, sex, and treatment effect; using time to cardiovascular endpoint as the dependent variable; and for all patients and for patients within each treatment group. First, OxPL-apoB was used as a continuous variable, with log2 transformation. Then, OxPL-apoB was ranked into tertiles and expressed as 3 dummy indicator variables using the 1st tertile as the control. Treatment by OxPL-apoB interaction was examined separately in the same model with the addition of interaction term. The proportional hazards assumption was examined and confirmed not violated by looking at the supremum test and the cumulative martingale residuals. All analyses were performed using SAS software, version 9.
In a simple Student t test with an observed standard deviation of 4.16 nmol/l PC-OxPL, a sample size of 1,500 has 80% power to detect a difference of 0.15 SD (0.62 nmol/l) between the 2 treatment groups, or a difference of 0.24 SD (1.00 nmol/l) between patients who did and did not experience a primary endpoint event during the study, given the event rate of 10%. Alternatively, since PC-OxPL is log-transformed before analyses, on a log2 scale, the SD is 1.043. Using a Student t test based on a log-transformed data, a sample size of 1,500 has 80% power to detect an 11.5% difference between the 2 treatment groups, or 19.1% difference between patients who did and did not experience a primary endpoint event during the study, given the event rate of 10%.
RESULTS
The characteristics of patients in the main study and in the biomarker substudy at the time of randomization are shown in Table 1. In the biomarker study 1,503 patients were included, of whom 754 were treated with atorvastatin 10 mg and 749 with atorvastatin 80 mg. Of these subjects, 156 had a MACE and 1,347 had no event. Patients with MACE were older and characterized by more hypertension, diabetes, current smokers, and lower high-density lipoprotein cholesterol (HDL-C), but similar LDL-C and Lp(a) levels. At randomization, OxPL-apoB levels were not significantly different between atorvastatin 10 mg and atorvastatin 80 mg. However, patients with events had higher levels (median interquartile range [Q1, Q3]) of OxPL-apoB at randomization than those without events 4.4 (2.7, 7.9) versus 3.7 (2.3, 6.8) nmol/l PC-OxPL (p = 0.025; Table 1). These findings on OxPL-apoB were confirmed in a Student t test based on a log-transformed OxPL-apoB. Patients with events had 14.5% higher OxPL-apoB level, or 0.195 higher on a log2 scale, than those without events (p = 0.027). No significant difference was observed between treatment groups.
TABLE 1.
Patient Characteristics at Randomization*
| Main Study | Biomarker Substudy | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | By Treatment | All Patients | By Treatment | By Event | ||||
| ATV 10 mg (n = 5,006) |
ATV 80 mg (n = 4,995) |
(n = 1,503) | ATV 10 mg (n = 754) |
ATV 80 mg (n = 749) |
With Event (n = 156) |
Without Event (n = 1,347) |
p Value† | |
| Age, yrs | 60.9 (8.8) | 61.2 (8.8) | 61.5 (8.7) | 61.3 (8.5) | 61.8 (8.9) | 62.8 (8.5) | 61.4 (8.7) | 0.034 |
| Male, % | 80.8 | 81.2 | 78.8 | 80.1 | 79.7 | 83.3 | 78.3 | 0.14 |
| Risk factor | ||||||||
| Current smoker, % | 13.4 | 13.4 | 12.4 | 14.6 | 11.2 | 18.6 | 11.7 | 0.034 |
| Hypertension, % | 54.4 | 53.9 | 57.7 | 58.4 | 55.9 | 69.2 | 56.4 | 0.002 |
| Diabetes, % | 15.0 | 15.0 | 16.6 | 16.9 | 16.3 | 26.3 | 15.5 | 0.0006 |
| Lipids | ||||||||
| LDL cholesterol, mg/dl | 98 (18) | 98 (17) | 98 (18) | 98 (18) | 97 (18) | 98 (18) | 98 (18) | 0.91 |
| Total cholesterol, mg/dl | 175 (24) | 175 (24) | 175 (24) | 175 (23) | 175 (25) | 175 (23) | 175 (24) | 0.96 |
| Triglycerides, mg/dl | 134 (101, 184) | 135 (102, 183) | 138 (106, 189) | 142 (106, 191) | 136 (106, 185) | 139 (110, 200) | 138 (105, 187) | 0.27 |
| HDL cholesterol, mg/dl | 47 (11) | 47 (11) | 47 (11) | 46 (11) | 47 (11) | 44 (10) | 47 (11) | 0.0007 |
| Lp(a), mg/dl | -- | -- | 15 (5, 39.5) | 15 (5, 42) | 14 (5, 37) | 20 (5, 47.5) | 14 (5, 38) | 0.104 |
| OxPL-apoB, nmol/l | -- | -- | 3.8 (2.4, 6.9) | 3.8 (2.4, 7.0) | 3.7 (2.3, 6.9) | 4.4 (2.7, 7.9) | 3.7 (2.3, 6.8) | 0.025 |
Values are mean (SD) or median (interquartile range) unless otherwise indicated.
At the time of randomization, all participants had received atorvastatin 10 mg for 8 weeks.
p value for patients who experienced an event versus those who did not in the biomarker subgroup.
ATV = atorvastatin; HDL = high-density lipoprotein; LDL = low-density lipoprotein; Lp(a) = lipoprotein(a); OxPL-apoB = oxidized phospholipids on apolipoprotein B-100.
In the overall cohort, the level of OxPL-apoB at randomization (after the 8-week run-in period with atorvastatin 10 mg) predicted recurrent events. The HR associated with doubling of the OxPL-apoB concentration was 1.20 (95% CI: 1.03 to 1.39; p = 0.018) after adjusting for age, sex, and treatment (Table 2). The difference in the atorvastatin 10 mg group remained significant (HR: 1.23; 95% CI: 1.00 to 1.51; p = 0.05), whereas in the atorvastatin 80 mg group it lost significance (HR: 1.17; 95% CI: 0.94 to 1.46; p = 0.16). Treatment interaction by OxPL-apoB levels at randomization did not reach statistical significance, likely due to the fact that the test of interaction often requires a much larger sample size. The 1-year OxPL-apoB levels and the change from randomization to year 1 in OxPL-apoB levels were not associated with higher risk of recurrent events for the overall, atorvastatin 10 mg, or atorvastatin 80 mg groups.
TABLE 2.
Association Between Randomization, Year 1, Change from Randomization to Year 1 in OxPL-apoB and Clinical Outcomes*
| All Patients | Atorvastatin 10 mg | Atorvastatin 80 mg | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR† | 95% CI | p Value | HR† | 95% CI | p Value | HR† | 95% CI | p Value | |
| Randomization | 1.20 | 1.03, 1.39 | 0.018 | 1.23 | 0.99, 1.51 | 0.053 | 1.17 | 0.94, 1.46 | 0.156 |
| Year 1 | 1.15 | 0.96, 1.38 | 0.143 | 1.22 | 0.95, 1.58 | 0.127 | 1.06 | 0.81, 1.38 | 0.670 |
| Change from randomization to year 1 | 0.93 | 0.74, 1.17 | 0.523 | 0.95 | 0.68, 1.31 | 0.736 | 0.90 | 0.65, 1.23 | 0.503 |
At the time of randomization, all participants had received 10mg atorvastatin for 8 weeks.
Hazard ratio (HR) associated with doubling the concentration and adjusting for age, sex, and treatment effect. Treatment interaction by individual biomarker is not significant for all biomarkers analyzed.
CI = confidence interval; other abbreviations as in Table 1.
The findings between randomization levels of OxPL-apoB and subsequent MACE remained consistent after further adjustment for all traditional risk factors including age, sex, BMI, diabetes mellitus, systolic blood pressure (SBP), LDL-C, HDL-C, ApoB, and treatment (HR: 1.21; 95% CI: 1.04 to 1.41; p = 0.018) per doubling of OxPL-apoB concentration in the total population. This remained significant in the atorvastatin 10 mg group (HR: 1.28; 95% CI: 1.03 to 1.58; p = 0.028), but not the atorvastatin 80 mg group (HR: 1.16; 95% CI: 0.93 to 1.44; p = 0.19)).
Evaluating groups by tertiles (Figure 1), tertile 3 was associated with a higher risk of MACE compared to the first tertile in the overall group (HR: 1.69; 95% CI: 1.14 to 2.49; p = 0.01) and in the atorvastatin 10 mg group (HR: 2.08; 95% CI: 1.20 to 3.61; p = 0.01). This was not significant in the atorvastatin 80 mg group (HR: 1.40; 95% CI: 0.80 to 2.46; p = 0.24). The treatment by OxPL-apoB interaction was not significant (p = 0.37).
FIGURE 1. Association Between OxPL-apoB Levels at Randomization and Subsequent Major CV Events.
Hazard ratios versus tertile 1 were adjusted for age, sex, body mass index, diabetes mellitus, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, apoB, and difference between treatment groups. Within each panel, the p values refer to comparisons of tertile 2 or 3 to tertile 1. ATV = atorvastatin; OxPL-apoB = oxidized phospholipids on apolipoprotein B-100.
To test the robustness of our results, we also performed a logistic regression analysis looking at the proportion of patients within the total study population who experienced a MACE during the study. After adjusting for age, sex, and treatment group, OxPL-apoB significantly predicted MACE as expected. The odds ratio (OR) associated with doubling the concentration of OxPL-apoB was 1.21 (95% CI: 1.03 to 1.42; p = 0.021). A receiving operating characteristics (ROC) analysis was also performed, showing that the area under the ROC curve increased from 0.5735 to 0.5868 by adding OxPL-apoB to the model. However, this increase of 0.0133 was not statistically significant (95% CI: −0.0175 to 0.0440; p = 0.39). The incremental area under ROC remained nonsignificant when it was added to the model of other traditional risk factor variables, including baseline levels of LDL-C, HDL-C, apoB, SBP, BMI, and diabetes mellitus. Nevertheless, this nonsignificance of incremental area under the ROC curve does not negate the potential prognostic value of OxPL-apoB for subsequent MACE since the well-established traditional risk factors also failed to show a significant incremental area under ROC to predict MACE when analyzed in the same model. However, like OxPL-apoB, traditional risk factors including age, BMI, diabetes mellitus, SBP, and HDL-C were all significant predictors in both the Cox proportional hazard and the logistic regression analyses, but failed to achieve significant incremental area under ROC. Further research is needed to validate the utility of the ROC analytical approach in such circumstances. Likely due to their strong correlation (see below), neither OxPL-apoB nor Lp(a) were significant predictors when both were included in the Cox proportional hazard analysis. We had previously reported the prognostic value of OxPL-apoB in primary prevention settings by showing that it reclassifies ~30% of patients from the general community using c-statistics and net reclassification measures (17).
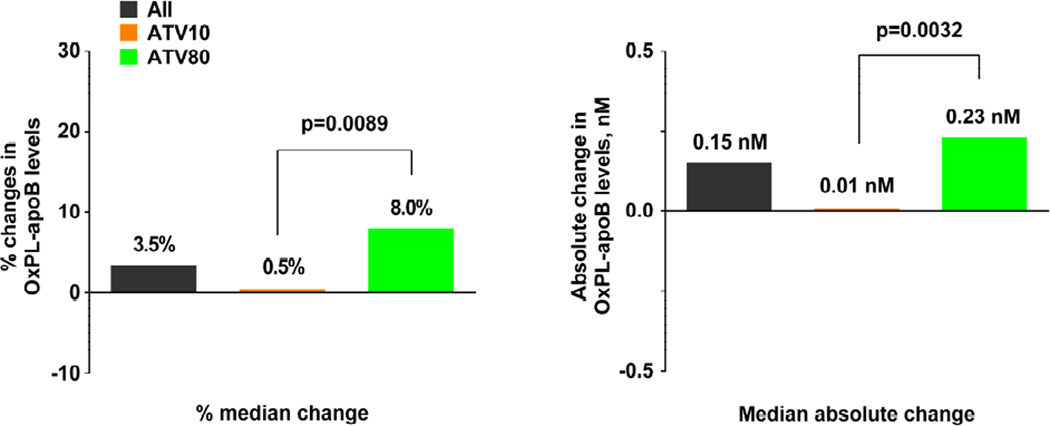
In the overall cohort, the median percent change in OxPL-apoB levels from randomization to 1 year was 3.5% (interquartile range [IQR]: −28.9% to +45.1%; Figure 2A). In the atorvastatin 10 mg group, there was no significant change in the median percent change (0.5%; IQR: −30.8% to +36.7%; p = 0.061). However, in the atorvastatin 80 mg group, there was a significant increase in the median percent change at 8.0% (−IQR: −26.9% to +53.3%; p<0.0001). This treatment group difference was statistically significant (p = 0.0089). Similar findings were present in the changes of absolute levels of OxPL-apoB (Figure 2A).
FIGURE 2. Changes in OxPL-apoB Levels from Randomization to Year 1.
Both percent and absolute changes in OxPL-apoB levels are shown according to (A) treatment group and (B) whether the patient experienced a major cardiovascular (CV) event. The p values are from comparisons using the 2-sided Wilcoxon rank sum test. Other abbreviations as in Figure 1.
In patients with recurrent events, the median percent change in OxPL-apoB levels from randomization to 1 year was −7.6% (IQR: −35.5% to +49.5%; p = 0.34; Figure 2B). In patients who did not experience an event, there was a significant increase in the median change at 5.0% (IQR: −27.7% to +43.8%; p <0.0001). However, the differences between patients who did and did not experience an event were not significant (p = 0.24). Similar findings were present in the changes of absolute levels of OxPL-apoB (Figure 2B).
Spearman correlation analyses were carried out between randomization and 1-year OxPL-apoB as well as with Lp(a), which have been previously shown to correlate with each other (18). OxPL-apoB at randomization correlated with 1-year OxPL-apoB (r = 0.65; p < 0.0001) and Lp(a) at randomization correlated with 1-year Lp(a) (r = 0.94; p < 0.0001). OxPL-apoB correlated with Lp(a) at randomization (r = 0.73; p < 0.0001) and 1-year Lp(a) (r = 0.71; p < 0.0001) (Figure 3). Weak to absent correlations were noted with lipid and laboratory variables in Table 1 (data not shown).
FIGURE 3. Spearman Correlation Analysis of OxPL-apoB and Lp(a).
Correlation between OxPL-apoB and lipoprotein(a) (Lp[a]) is shows (A) at randomization according to treatment; (B) at randomization according to whether the patient experienced a major CV event; (C) at 1 year according to treatment; and (D) at 1 year according to whether the patient experienced a major CV event. Other abbreviations as in Figures 1 and 2.
DISCUSSION
This TNT trial substudy demonstrates that elevated levels of OxPL-apoB predict secondary MACE in subjects with stable CHD. This was shown despite the fact that under this protocol there was no placebo arm and all patients were treated with atorvastatin 10 mg for 8 weeks prior to randomization, which has known effects on OxPL-apoB (8). Importantly, it suggests that high-dose treatment with atorvastatin mitigates the risk conferred by OxPL-apoB. This study expands the database of OxPL-apoB predicting CVD and clinical outcomes in primary prevention settings to statin-treated patients with established CHD in the context of a contemporary randomized clinical trial (Central Illustration).
CENTRAL ILLUSTRATION. Oxidized Phospholipids, Statin Therapy, and CV Outcomes.
Upper panel: The bar graph depicts the hazard or odds ratio of oxidized phospholipids on apolipoprotein B-100 (OxPL-apoB) for the primary endpoint in each study indicated, comparing the highest to the lowest tertile or quartile. These studies included subjects without or with prior cardiovascular disease (CVD), defined as myocardial infarction, stroke, or peripheral arterial disease The data are derived from prior publications including the Bruneck Study (17), EPIC (European Prospective Investigation of Cancer)-Norfolk Trial (9), Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study (HPFS) (38), Mayo Study (11) and Treating to New Targets (TNT) Trial. The Mayo Study (11) reflect odds ratio for angiographically-determined coronary artery disease.
Lower panel: The illustration depicts the risk of CVD mediated by increased circulating levels of OxPL-apoB in the context of intensity of statin therapy in patients with subclinical atherosclerosis, angiographically-determined CAD, and in established CAD by clinical criteria. As the intensity of statin therapy increases, the risk of new events (denoted a ruptured plaque on the cartoon) mediated by OxPL-apoB decreases but is not completely abrogated.
It is well accepted that innate and adaptive immune mechanisms modulate atherosclerosis. Immune responses to OSEs, such as OxPLs, play a central role in the development of atherosclerosis (2). OxPLs are pro-atherogenic and upregulate pro-inflammatory molecules in macrophages and endothelial cells (5). OxPL are present on Lp(a), both in the lipid phase and covalently bound to apolipoprotein (a) (apo(a), and the kringle IV type 10 segment of apo(a) strongly influences their binding (6,19). OxPLs mediate macrophage apoptosis, a key component of plaque vulnerability, in endoplasmic reticulum-stressed macrophages by signaling through the CD36/Toll-like receptor-2 pathway (20). Additionally, monocyte chemoattractant protein-1 (MCP-1) binds to OxPL on Lp(a) in human plasma, and once Lp(a) is retained in the vessel wall, MCP-1 retains its ability to recruit monocytes, thus promoting enhanced trafficking into the vessel wall (21). Finally, apo(a) and OxPL epitopes are significantly enriched in pathologically-defined human vulnerable plaques and clinically-relevant lesions that require intervention, consistent with a clinically relevant pathological role in the expression of CVD and events (3,13,14,22).
An extensive literature demonstrates that elevated plasma levels of OxPL-apoB predict the presence and progression of coronary, carotid, and femoral/peripheral arterial disease; predict death, MI, and stroke in subjects from the general community; and reclassify ~30% of patients into different risk categories (11). Lp(a) is also the major lipoprotein carrier of OxPLs, which may impart pro-inflammatory properties (6,7). The risk of Lp(a) is strongly associated with its content of OxPLs, which in turn are greatly enriched in small apo(a) isoforms that are associated with the highest plasma Lp(a) levels (18). Indeed, elevated plasma levels of OxPL-apoB are associated with acute coronary syndromes and increase post percutaneous coronary interventions (23,24).
Several therapeutic interventions increase OxPL-apoB levels, including low-fat diets, garlic supplements, and most statins, as noted here in the TNT Trial; we emphasize that these are absolute levels and not a ratio of OxPL to apoB levels measured in plasma. The initial hypothesis was that OxPL-apoB and Lp(a) levels would increase during hypercholesterolemia and atherosclerosis progression and would decrease during atherosclerosis regression in animals. Similarly, it was thought that OxPL-apoB and Lp(a) levels would decrease during therapies that caused regression in animal models or during low-fat diets in humans. Surprisingly, and paradoxically, we have observed the opposite to date, namely, increases in plasma OxPL-apoB in New Zealand white rabbits and cynomolgus monkeys on regression diets (25). Similarly, we observed increases in both OxPL-apoB and its carrier Lp(a) in humans on low-fat diets (26,27) or Step II American Heart Association diets (28), or following statin therapy (28–33) or administration of aged garlic supplements in subjects with CAD on a background of statin therapy (34,35).
These data demonstrate that the increase in OxPL-apoB and Lp(a) that occurred in response to various interventions was associated with regression of atherosclerosis in experimental models and with improvement in vascular function and reduced rate of progression of coronary calcification in humans (34,35). Although the mechanisms of the increase in OxPL-apoB in plasma in response to effective therapy are not understood yet, immunostaining of atherosclerotic lesions for their content of OxPL at baseline and after dietary-induced lesion regression in New Zealand white rabbits and cynomolgus monkeys showed loss of OxPL in atherosclerotic plaques concomitantly with an increase in OxPL-apoB levels in the plasma. This is consistent with an efflux of OxPL from the vessel wall to the circulation, where it was bound to apoB-containing lipoproteins such as Lp(a), and suggest the hypothesis of a potential stabilization or regression of lesions (25). Presumably, if one followed such subjects for sufficient periods of time in the future, the OxPL-apoB levels would revert to lower levels, thus explaining the ability of baseline OxPL-apoB levels to predict cardiovascular outcomes. A second possibility is that OxPL-apoB may increase along with Lp(a) increases induced by statin therapy (8). Interestingly, if one directly reduces Lp(a) in plasma, as has been shown recently with antisense therapy to apo(a) in both transgenic-Lp(a) mice (36) and humans (37), then OxPL-apoB levels decrease substantially and in proportion to the decrease in the carrier Lp(a).
STUDY LIMITATIONS
This is a TNT substudy and the number of events was modest (n = 156) compared to the overall trial (n = 982) (15). Blood samples were not available prior to randomization and it is likely that 8 weeks of atorvastatin therapy affected the levels of OxPL-apoB, which may have attenuated its full predictive power.
CONCLUSIONS
OxPL-apoB levels are elevated in patients with subsequent MACE and these levels also predict secondary MACE in subjects with stable CHD, a risk that is mitigated by high-dose (80 mg) atorvastatin. Elevated levels of OxPL-apoB appear to be a clinically informative biomarker in predicting MACE in secondary care prevention settings.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge
Patients with cardiovascular disease who have elevated levels of pro-inflammatory oxidized phospholipids on apoB-containing lipoproteins are a high risk of ischemic events. High-dose statin therapy, but not low-dose therapy, reduces the risk mediated by oxidized phospholipids.
Translational Outlook
Future studies should assess the value of measuring oxidized phospholipid levels on apoB-containing lipoproteins to risk-stratify patients for ischemic events and guide statin dosing.
Acknowledgments
Assistance in preparing the tables and figures was provided by Shuang Li, PhD at Engage Scientific Solutions and was funded by Pfizer Inc.
Funding Sources: This investigation was supported by the General Clinical Research Center, University of California, San Diego (UCSD) with funding provided by the National Center for Research Resources, M01RR00827, USPHS. Drs. Tsimikas and Witztum are also supported by NIH (NHLBI) grants R01-HL119828, R01-HL093767, P01-HL088093 and P01-HL055798. Dr. Byun was supported by the Inje Research and Scholarship Foundation in 2013. Pfizer, Inc. supported the cost of the assays through a laboratory service agreement with UCSD.
Disclosure: Drs. Tsimikas and Witztum are co-inventors and receive royalties from patents owned by UCSD on oxidation-specific antibodies and of biomarkers related to oxidized lipoproteins. Dr. Tsimikas currently has a dual appointment with UCSD and as an employee of Isis Pharmaceuticals. Dr. Witztum is a consultant to ISIS Pharmaceuticals, Regulus, CymaBay and Intercept. Drs Bao, DeMicco and Laskey are employees of Pfizer, Inc.
ABBREVIATIONS
- CHD
coronary heart disease
- LDL-C
low-density lipoprotein cholesterol
- Lp(a)
lipoprotein(a)
- MACE
major adverse cardiac events
- OSE
oxidation-specific epitope
- OxPL-apoB
oxidized phospholipids on apolipoprotein B-100
- PC-OxPL
phosphocholine-containing oxidized phospholipids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Witztum JL, Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol. 2014;9:73–102. doi: 10.1146/annurev-pathol-020712-163936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dijk RA, Kolodgie F, Ravandi A, et al. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J Lipid Res. 2012;53:2773–2790. doi: 10.1194/jlr.P030890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee S, Birukov KG, Romanoski CE, Springstead JR, Lusis AJ, Berliner JA. Role of phospholipid oxidation products in atherosclerosis. Circ Res. 2012;111:778–799. doi: 10.1161/CIRCRESAHA.111.256859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadl A, Sharma PR, Chen W, et al. Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Rad Biol Med. 2011;51:1903–1909. doi: 10.1016/j.freeradbiomed.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmark C, Dewan A, Orsoni A, et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–2239. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Leibundgut G, Scipione C, Yin H, et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a) J Lipid Res. 2013;54:2815–2830. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taleb A, Witztum JL, Tsimikas S. Oxidized phospholipids on apolipoprotein B-100 (OxPL/apoB) containing lipoproteins: A biomarker predicting cardiovascular disease and cardiovascular events. Biomarkers Med. 2011;5:673–694. doi: 10.2217/bmm.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsimikas S, Mallat Z, Talmud PJ, et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol. 2010;56:946–955. doi: 10.1016/j.jacc.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 10.Tsimikas S, Hall JH. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: A rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J Am Coll Cardiol. 2012;60:716–721. doi: 10.1016/j.jacc.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 11.Tsimikas S, Duff GW, Berger PB, et al. Pro-inflammatory interleukin-1 genotypes potentiate the risk of coronary artery disease and cardiovascular events mediated by oxidized phospholipids and lipoprotein(a) J Am Coll Cardiol. 2014;63:1724–1734. doi: 10.1016/j.jacc.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman P, Horkko S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J Biol Chem. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 13.Choi SH, Yin H, Ravandi A, et al. Polyoxygenated cholesterol ester hydroperoxide activates TLR4 and SYK dependent signaling in macrophages. PLoS One. 2013;8:e83145. doi: 10.1371/journal.pone.0083145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravandi A, Leibundgut G, Hung MY, et al. Release and capture of bioactive oxidized phospholipids and oxidized cholesteryl esters during percutaneous coronary and peripheral arterial interventions in humans. J Am Coll Cardiol. 2014;63:1961–1971. doi: 10.1016/j.jacc.2014.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaRosa JC, Grundy SM, Waters DD, et al. Intensive Lipid Lowering with Atorvastatin in Patients with Stable Coronary Disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 16.Arsenault BJ, Barter P, DeMicco DA, et al. Prediction of cardiovascular events in statin-treated stable coronary patients of the treating to new targets randomized controlled trial by lipid and non-lipid biomarkers. PLoS One. 2014;9:e114519. doi: 10.1371/journal.pone.0114519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsimikas S, Willeit P, Willeit J, et al. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60:2218–2229. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- 18.Tsimikas S, Clopton P, Brilakis ES, et al. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: results from the Dallas Heart Study. Circulation. 2009;119:1711–1719. doi: 10.1161/CIRCULATIONAHA.108.836940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leibundgut G, Witztum JL, Tsimikas S. Oxidation-specific epitopes and immunological responses: Translational biotheranostic implications for atherosclerosis. Curr Opin Pharmacol. 2013;13:168–179. doi: 10.1016/j.coph.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seimon TA, Nadolski MJ, Liao X, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiesner P, Tafelmeier M, Chittka D, et al. MCP-1 binds to oxidized LDL and is carried by lipoprotein(a) in human plasma. J Lipid Res. 2013;54:1877–1883. doi: 10.1194/jlr.M036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purushothaman KR, Purushothaman M, Levy AP, et al. Increased expression of oxidation-specific epitopes and apoptosis are associated with haptoglobin genotype: possible implications for plaque progression in human atherosclerosis. J Am Coll Cardiol. 2012;60:112–119. doi: 10.1016/j.jacc.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Tsimikas S, Bergmark C, Beyer RW, et al. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41:360–370. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- 24.Tsimikas S, Lau HK, Han KR, et al. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109:3164–3170. doi: 10.1161/01.CIR.0000130844.01174.55. [DOI] [PubMed] [Google Scholar]
- 25.Tsimikas S, Aikawa M, Miller FJ, Jr, et al. Increased plasma oxidized phospholipid:apolipoprotein B-100 ratio with concomitant depletion of oxidized phospholipids from atherosclerotic lesions after dietary lipid-lowering: a potential biomarker of early atherosclerosis regression. Arterioscler Thromb Vasc Biol. 2007;27:175–181. doi: 10.1161/01.ATV.0000251501.86410.03. [DOI] [PubMed] [Google Scholar]
- 26.Faghihnia N, Tsimikas S, Miller ER, Witztum JL, Krauss RM. Changes in lipoprotein(a), oxidized phospholipids, and LDL subclasses with a low-fat high-carbohydrate diet. J Lipid Res. 2010;51:3324–3330. doi: 10.1194/jlr.M005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silaste ML, Rantala M, Alfthan G, et al. Changes in dietary fat intake alter plasma levels of oxidized low-density lipoprotein and lipoprotein(a) Arterioscler Thromb Vasc Biol. 2004;24:498–503. doi: 10.1161/01.ATV.0000118012.64932.f4. [DOI] [PubMed] [Google Scholar]
- 28.Rodenburg J, Vissers MN, Wiegman A, et al. Oxidized low-density lipoprotein in children with familial hypercholesterolemia and unaffected siblings: effect of pravastatin. J Am Coll Cardiol. 2006;47:1803–1810. doi: 10.1016/j.jacc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 29.Tsimikas S, Witztum JL, Miller ER, et al. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation. 2004;110:1406–1412. doi: 10.1161/01.CIR.0000141728.23033.B5. [DOI] [PubMed] [Google Scholar]
- 30.Ky B, Burke A, Tsimikas S, et al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J Am Coll Cardiol. 2008;51:1653–1662. doi: 10.1016/j.jacc.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Choi SH, Prasad A, Tsimikas S. The evolution of thienopyridine therapy clopidogrel duration, diabetes, and drug-eluting stents. J Am Coll Cardiol. 2008;51:2228–2229. doi: 10.1016/j.jacc.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Fraley AE, Schwartz GG, Olsson AG, et al. Relationship of oxidized phospholipids and biomarkers of oxidized low-density lipoprotein with cardiovascular risk factors, inflammatory biomarkers, and effect of statin therapy in patients with acute coronary syndromes: Results from the MIRACL (Myocardial Ischemia Reduction With Aggressive Cholesterol Lowering) trial. J Am Coll Cardiol. 2009;53:2186–2196. doi: 10.1016/j.jacc.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida H, Shoda T, Yanai H, et al. Effects of pitavastatin and atorvastatin on lipoprotein oxidation biomarkers in patients with dyslipidemia. Atherosclerosis. 2013;226:161–164. doi: 10.1016/j.atherosclerosis.2012.10.069. [DOI] [PubMed] [Google Scholar]
- 34.Budoff MJ, Ahmadi N, Gul KM, et al. Aged garlic extract supplemented with B vitamins, folic acid and L-arginine retards the progression of subclinical atherosclerosis: a randomized clinical trial. Prev Med. 2009;49:101–107. doi: 10.1016/j.ypmed.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Ahmadi N, Tsimikas S, Hajsadeghi F, et al. Relation of oxidative biomarkers, vascular dysfunction, and progression of coronary artery calcium. Am J Cardiol. 2010;105:459–466. doi: 10.1016/j.amjcard.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 36.Merki E, Graham M, Taleb A, et al. Antisense oligonucleotide lowers plasma levels of apolipoprotein (a) and lipoprotein (a) in transgenic mice. J Am Coll Cardiol. 2011;57:1611–1621. doi: 10.1016/j.jacc.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 37.Viney NJ, Graham M, Crooke R, Hughes S, Singleton W. Evaluation of Isis apo(a)Rx, an antisense inhibitor to apolipoprotein(a), in healthy volunteers. Circulation. 2013;128:A14196. [Google Scholar]
- 38.Bertoia ML, Pai JK, Lee JH, et al. Oxidation-specific biomarkers and risk of peripheral artery disease. J Am Coll Cardiol. 2013;61:2169–2179. doi: 10.1016/j.jacc.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.