Abstract
Background:
Diabetes is associated with micro- and macro-vascular complications affecting several organs. Oxidative stress plays a crucial role in the etiology of vascular disease in diabetes.
Objectives:
The present study aimed to investigate the beneficial effect of troxerutin on diabetes-induced histopathological damages in rat aorta with focusing on its antioxidative actions.
Materials and Methods:
Male Wistar rats were randomly divided into four groups (n = 8/each): control, control plus troxerutin, diabetic and diabetic plus troxerutin. Diabetes was induced in rats by a single intraperitoneal injection of streptozotocin (50 mg/kg) and lasted for 10 weeks. Troxerutin was administered orally in concentration of 150 mg/kg/daily for one month before killing rats. At the end of treatment period, thoracic aorta was isolated and divided into two parts; one part was immersed in 10% formalin for histopathological evaluations and the other was frozen by liquid nitrogen for assessment of malondialdehyde (MDA, the main product of lipid peroxidation), activity of antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPX).
Results:
Lipid deposition in tunica intimae and media, thickening and structural deformity of vascular tissues as well as the level of plasma glucose and aortic tissue levels of lipid peroxidation were significantly increased in diabetic rats compared to control ones (P < 0.05). Troxerutin significantly reduced the severity of all vascular histopathological damages in treated versus untreated diabetic rats. In addition, treatment of diabetic rats with troxerutin significantly decreased the levels of MDA (5.1 ± 0.3 vs. 9.3 ± 1.2 nmol/mL) (P < 0.01) and increased the activity of antioxidant enzyme GPX compared to untreated-diabetic groups.
Conclusions:
Troxerutin may reduce the vascular complications and tissue injuries induced by chronic diabetes in rat aorta through increasing the activity of tissue antioxidant system and reducing the level of lipid peroxidation.
Keywords: Aorta, Diabetes, Troxerutin, Vascular Disease, Oxidative Stress
1. Background
Diabetes is considered as an important risk factor for the development of cardiovascular complications and vascular diseases, which are considered as the leading cause of mortality in individuals with diabetes (1-3). It has been suggested that both conduit and resistance arteries such as aorta are not functional in diabetes and impairment of endothelial function underlies both micro- and macro-vascular complications of diabetes (4, 5). The underlying mechanisms of these complications are complex; however, it is believed that the production of reactive oxygen species (ROS) and oxidative stress play an important role (6-8). Lipid peroxidation and overproduction of ROS including O2- and H2O2 have been reported in aorta of diabetic rats, leading to formation of cell membrane-damaging compounds and glycosylation of proteins which have been associated with vascular dysfunctions in diabetes (3, 5, 9).
Using new potent therapeutic regimens containing natural materials in the treatment of metabolic complications can be interesting because of their multipotent potentials, safety and lower adverse effects (10). Troxerutin, also known as vitamin P4, is a tri-hydroxyethylated derivative of natural bioflavonoid rutins found in tea, coffee, cereals, fruits and vegetables (11, 12). It can be easily absorbed by gastrointestinal system and has a variety of biological activities including anti-oxidative, anti-inflammatory and anti-thrombolytic properties (13, 14). Previous experiments confirmed tissue protective effect of troxerutin in kidney (15), liver (16) and brain (17, 18) injuries. Furthermore, this material is used in the treatment of varicose veins, chronic venous deficiency and edemas due to traumatic veins (19). However, to our knowledge, it has not yet been reported that troxerutin could attenuate vascular damage in diabetic models.
2. Objectives
Regarding its potential effects in controlling cardiovascular diseases and diabetes, and considering the antioxidant potential of this material, the aim of this study was to evaluate whether troxerutin affect diabetes-induced aortic damages and to explore its mechanism of action. Therefore, we hypothesized that troxerutin might reduce aortic histopathological damages induced by diabetes through attenuating oxidative stress and suppressing lipid peroxidation.
3. Materials and Methods
3.1. Animals and Materials
Thirty-two adult male Wistar rats weighing 250-300 grams were used in this study. Animals were kept in polypropylene cages and experienced a 12-hour light/12-hour dark, 50% humidity and temperature of 25°C and had free access to food and water. This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No 85-23, revised 1996) and was approved by the local university animal care committee. Troxerutin was purchased from Sigma (St. Louis, MO, USA) and all other chemicals and reagents were obtained from commercial sources in the highest quality available.
3.2. Experimental Design and Induction of Diabetes
The animals were randomly divided into four groups (n = 8/each) as: group I: control (C), group II: control with troxerutin (C + TXR), group III: diabetic (D), and group IV: diabetic with troxerutin (D + TXR). Diabetes was induced by a single intraperitoneal (ip) injection of streptozotocin (STZ; 50 mg/kg; dissolved in 0.1 molar citrate buffer with pH 4.5). The control rats were received the same amount of citrate buffer alone. Development of diabetes was confirmed by measuring blood glucose levels, 72 hours later. Animals with blood glucose levels higher than 16.65 mmol/L (300 mg/dL) were considered diabetic and those with blood glucose levels lower than this value were excluded from the experiment (20, 21). After six weeks of STZ injection, troxerutin (150 mg/kg/day) was administered orally, once daily for four weeks (22-24). After 10 weeks of induction of diabetes, diabetic animals as well as the time-matched controls were killed and aortic samples were collected.
3.3. Sampling and Tissue Processing
After overnight food deprivation, blood glucose of rats was measured using a glucometer and then the animals were anesthetized with an intraperitoneal injection of ketamine/xylazine (60/10 mg/kg). The thoracic aorta was immediately removed and rinsed in a cold saline and weighed. The aortic tissue was divided into two parts; one part was frozen by liquid nitrogen and stored in a -80 °C refrigerator until biochemical assays and the other part was immersed in 10% formalin for histopathological evaluations.
3.4. Histopathological Assessment of the Aorta
For histopathological studies, some parts of the aortic tissue was isolated and placed in 10% formalin solution, dehydrated in ethanol and cleared in xylene and embedded in paraffin. After tissue processing steps, several serial sections of aorta (5 µm thicknesses) were prepared and stained by Hematoxylin & Eosin (H&E) for microscopic observations and studies. The thickness of media tunica was measured using Motic Images version 2.0 and light microscope. The stained sections were also evaluated by a blind histologist for subendothelial thickness, deposition of lipids in tunica intimae and media and morphology, density of the smooth muscle cells and changes were quantified according to the 4-grade scoring schedules (Table 1).
Table 1. Grading Systems for Evaluation of Lipid Deposition in Intimae and Media Tunica and Evaluation of Density of Smooth Muscle Cells (SMCs) Nuclei.
| Grading Systems | Evaluation | Grade |
|---|---|---|
| Evaluation of lipid deposition in intimae and media | ||
| Less lipid existence in the artery | + | 1 |
| Moderate lipid existence in the artery | ++ | 2 |
| Existence of lipid in most part of artery | +++ | 3 |
| Existence of lipid in all part of artery | ++++ | 4 |
| Evaluation of density of SMCs nuclei | ||
| Mild density SMC nuclei in the artery | + | 1 |
| Moderate density SMC nuclei in the artery | ++ | 2 |
| Severe density SMC nuclei in most part of artery | +++ | 3 |
| Extreme density SMC nuclei in all part of artery | ++++ | 4 |
3.5. Preparation of Tissue Homogenates
Aortic samples were homogenized in lysis buffer at pH 7.4 in the presence of protease inhibitor cocktail (104 mM AEBSF, 0.08 mM aprotinin, 2 mM leupeptin, 4 mM bestatin A and 1.4 mM E-64) (Sigma-Aldrich, St Louis, MO) with a Polytron PT-10/ST homogenizer. Then, the homogenate was centrifuged at 10000 rpm for 10 minutes at 4 °C. The supernatants were removed from the homogenates and quickly frozen at -80 °C until determination of lipid peroxidation marker and anti-oxidative enzymes. Protein concentration of supernatant was estimated using the Bradford technique.
3.6. Lipid Peroxidation Measurement: Malondialdehyde
Lipid peroxides are unstable and decompose to form a series of compounds including reactive carbonyl compounds. Polyunsaturated fatty acid peroxides generate malondialdehyde (MDA) and its measurement has been used as indicator of lipid peroxidation, which is analyzed by measuring thiobarbituric acid reactive substances (TBARS) in homogenates. Briefly, the samples (250 µL) were mixed with 1 mL 10% trichloroacetic acid (TCA) and 1 mL of 0.67% thiobarbituric acid. Then samples were heated in a boiling water bath for 15 minutes and then n-butyl-alcohol (2:1 v:v) was added to the solution. After centrifugation (3500 g, 5 minutes) at room temperature, TBARS was determined from the absorbance at 535 nm, using a spectrophotometer (Pharmacia Biotech; England) and the values obtained were expressed as nmol per 100 mg tissue protein (25, 26). The intra- and inter-assay coefficients of variations on measurements performed on the same sample were less than 5% and 7%, respectively.
3.7. Measurement of Superoxide Dismutase
Superoxide dismutase (SOD) activity was determined using a RANSOD kit (Randox Crumlin, UK). SOD activity was measured at 505 nm by a spectrophotometer. In this method, xanthine and xanthine oxidase were used to generate superoxide radicals able to react with 2-[4-iodophenyl]-3-[4-nitrophenol]-5-phenyl tetrazolium chloride (ITN) to form a red formazan dye. Concentrations of substrates were 0.05 mmol/L for xanthine and 0.025 mmol/L for ITN. SOD activity was measured by the degree of inhibition of this reaction. After calculating the percent of inhibition using related formula, SOD activity was calculated by comparing with the standard curve and was expressed as U/mg protein (20, 27). The inter-sample coefficient of variation and intra-assay variation were less than 8%.
3.8. Measurement of Glutathione Peroxidase
Glutathione peroxidase (GPX) activity was determined using a RANSEL kit (Randox Crumlin, UK). GPX catalyzes the oxidation of glutathione (at a concentration of 4 mmol/L) by cumene hydroperoxide. In the presence of glutathione reductase (at a concentration ≥ 0.5 units/L) and 0.28 mmol/L of NADPH, oxidized glutathione is immediately converted to the reduced form with concomitant oxidation of NADPH to NAD+. The decrease in absorbance at 340 nm (37°C) was measured using a spectrophotometer, then GPX concentration was calculated and expressed as U/mg protein (20, 27). The coefficient of variation for intra‐assay and inter-assay were 7% and 8%, respectively.
3.9. Statistical Analysis
All numerical data was reported as mean ± SD. The histopathological parameters were analyzed using non-parametric Kruskal-Wallis and differences of biochemical parameters between the groups were analyzed using one-way ANOVA followed by Tukey’s test. P value less than 0.05 was considered statistically significant.
4. Results
4.1. Histopathological Findings
Histopathological examination of the aortic sections in control group revealed some lesions in tunica intimae and tunica media. In tunica intimae, certain lipid deposition led to thickening of subendothelial layer along with endothelial denudation in some limited areas. Less lipid deposition and deformity of smooth muscle cells were visible in tunica media in control rats (Figure 1 A).
Figure 1. Micrographs of Aortic Tissue Sections Stained With H&E in Diabetic and Control Rats.
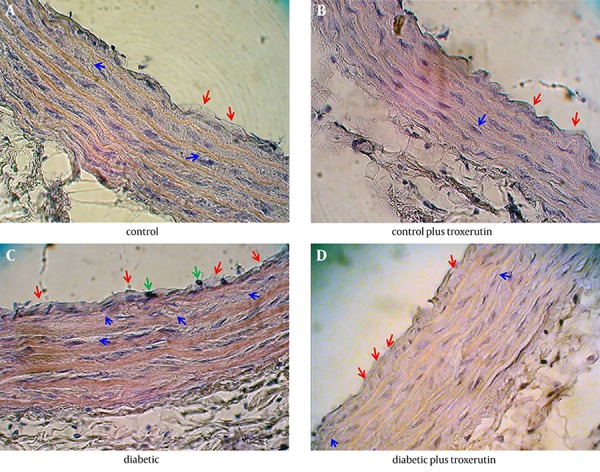
A, control group; B, control plus troxerutin group; C, diabetic group; D, diabetic plus troxerutin group. Red arrow indicates lipid deposition in tunica intimae; blue arrow indicates lipid deposition in tunica media and green arrow indicates macrophage infiltration in tunica intimae.
In aortic sections of control rats receiving troxerutin, the thickening of subendothelial layer and lipid deposition were lesser than those of control rats. The view of smooth muscle cells (SMCs) showed relatively normal morphology with normal arrangement of the fibers (Figure 1 B). Therefore, it seems that troxerutin may put some improving effects on the age-dependent aortic histological alterations in control rats (Table 2).
Table 2. Histopathological Parameters in Aortic Tissue of Diabetic and Control Rats and Effect of Troxerutin on Them a,b.
| Parameters | Control | C + TRX | Diabetes | D + TRX |
|---|---|---|---|---|
| Lipid deposition in intimae | 2.4 ± 0.4 | 1.2 ± 0.2 c | 4 ± 0.1 c | 3 ± 0.03 d |
| Lipid deposition in media | 2 ± 0.4 | 1.3 ± 0.2 | 3.6 ± 0.2 c | 3 ± 0.3 |
| SMCs density in media | 2 ± 0.3 | 1 ± 0.2 c | 3.2 ± 0.4 | 2.4 ± 0.2 d |
| Media thickness. µm | 395 ± 19 | 362 ± 29 | 547 ± 18 c | 422 ± 27 d |
a Abbreviations: C: control, D: diabetes, TXR: troxerutin, SMCs: smooth muscle cells.
b Data are presented as Mean ± SD.
c P < 0.05 as compared with control group.
d P < 0.05 as compared with diabetic group.
On the other hand, there was a more severe injury in the aortic tissue of diabetic rats compared with other groups, so that the lipid deposition in tunica intimae was extensive and voluminous. In addition, infiltration of macrophages-monocytes and endothelial denudation were substantial in tunica intimae of diabetic rats. The histopathological findings in tunica media of diabetic aorta were consisted of excessive lipid deposition, increased thickening of media along with disruption of media structure and deformity of SMCs with appearance of cells swelling and nuclear pyknosis (Figure 1 C).
Finally, in diabetic rats receiving troxerutin, the severity of histopathological alterations was relatively less than those of diabetic rats. Lipid depositions in tunica intimae and tunica media were attenuated in troxerutin-treated diabetic rats compared with untreated diabetic rats. Structural disarrangement and deformity of smooth muscle cells in aortic tissue of troxerutin-treated diabetic rats were considerably lower than histology of untreated diabetic aorta (Figure 1 D). Therefore, treatment of diabetic rats with troxerutin significantly reduced diabetes-induced aortic injury (Table 2).
4.2. Effect of Troxerutin on Plasma Glucose Level
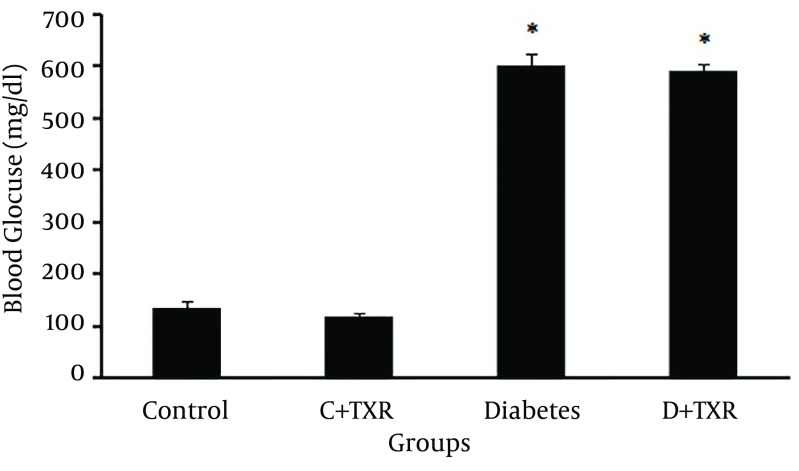
STZ administration in rats increased blood glucose levels significantly compared to control untreated rats. Orally administration of troxerutin in control rats had no significant effect on lowering blood glucose level. Although there was a reduction in blood glucose level of troxerutin-treated diabetic rats compared with untreated diabetic ones, this effect was not statistically significant (Figure 2).
Figure 2. Blood Glucose Level (mg/dL) in Diabetic and Control Rats and Effect of Troxerutin on It.
* P < 0.01 as compared with control group; C: control; D: diabetes; TXR: troxerutin.
4.3. Lipid Peroxidation Level in Experimental Groups
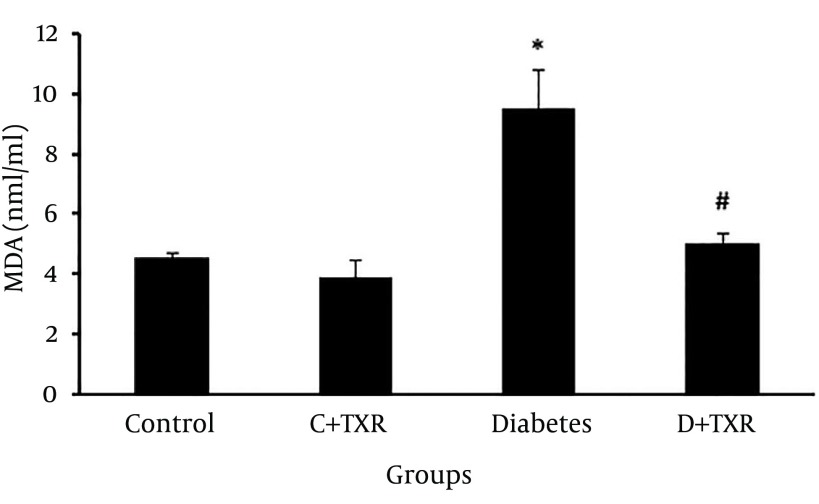
In assessment of MDA level as an index of lipid peroxidation, MDA level in diabetic group was significantly higher than that of controls (P < 0.05) (Figure 3). Administration of troxerutin for four weeks to diabetic rats significantly reduced the level of MDA compared to that of untreated diabetic rats (P < 0.01).
Figure 3. The Alteration of MDA Level (nm/mg Protein) in Diabetic and Control Rats and Effect of Troxerutin on it.
* P < 0.05 as compared with control group; # P < 0.01 as compared with diabetic group; C: control; D: diabetes; TXR: troxerutin.
4.4. Levels of Antioxidant Enzymes in Experimental Groups
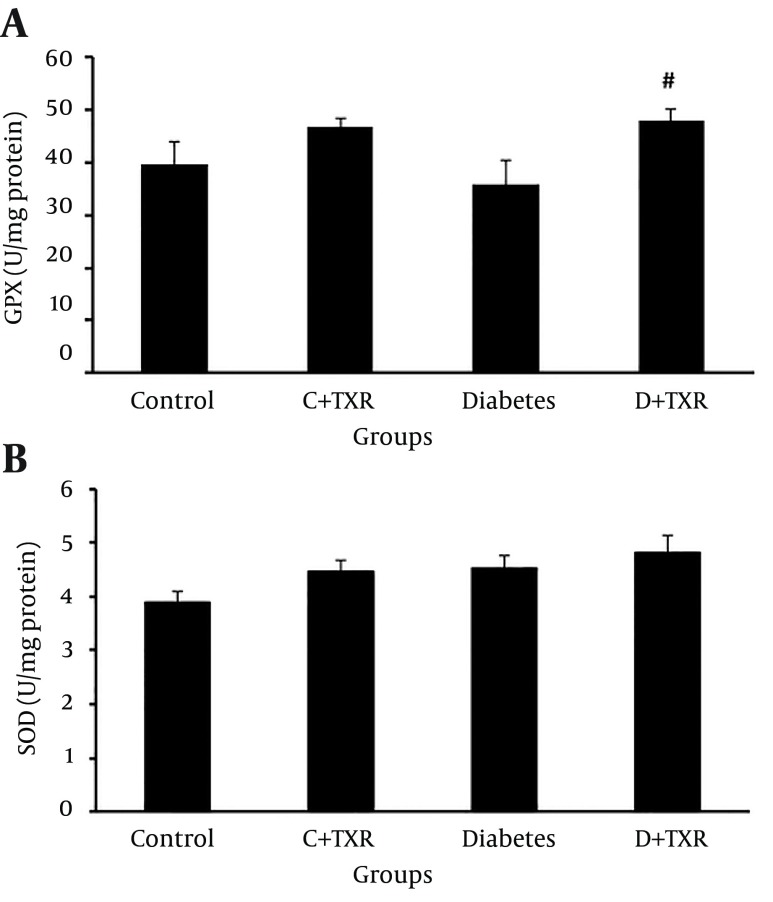
The findings of antioxidant enzymes (GPX and SOD) levels in four groups showed that administration of STZ in rats to induce diabetes could not significantly alter the levels of GPX (Figure 4 A) and SOD (Figure 4 B) in aortic tissue compared with control group. In addition, treatment of control rats with troxerutin had no statistically significant effect on these enzymes levels. However, administration of troxerutin in diabetic rats significantly increased the tissue level of antioxidant enzyme GPX compared with those of diabetic untreated rats (P < 0.1) (Figure 4).
Figure 4. Alteration of A, Antioxidant Enzymes GPX and B, SOD Levels (U/mg Protein) in Diabetic and Control Rats and Effect of Troxerutin on Them.
* P < 0.05 as compared with control group; # P < 0.01 as compared with diabetic group; C: control; D: diabetes; TXR: troxerutin.
5. Discussion
In this study, we investigated the effects of troxerutin administration on diabetic-induced aortic tissue injuries (as a macro-vasculature complication) along with its effects on oxidative stress levels. STZ-induced diabetes increased aortic tissue damages indicated by higher lipid deposition, SMCs density and thickening of blood vessel wall, and marked increased oxidative stress and lipid peroxidation indicated by increased MDA levels. Treatment with troxerutin significantly reduced aortic pathological damages induced by diabetes and this effect of troxerutin was accompanied with increased antioxidant enzyme (e.g. GPX) levels and reduced lipid peroxidation (MDA content) in aortic tissue of diabetic rats.
According to the findings of the present study, administration of troxerutin in dose of 150 mg/kg daily for four weeks could not significantly reduce blood glucose levels in type I diabetic rats, which is in accordance with the report of Chung et al. on the effect of troxerutin in STZ-induced diabetic retinopathy (28). On the other hand, Lu et al. found that troxerutin significantly reduced the level of blood glucose in type II diabetic rats induced by high cholesterol diet (18). Similarly, hypoglycemic effect of troxerutin (at the same dose) was recently reported by Geetha et al. in mice fed with high fat-fructose diet (22) and by Sampath et al. in sucrose-induced type II diabetic rats (23). This contrary possibly is related to the type of diabetic models used in different experiments. It can be supposed from these different findings that troxerutin may have no effect on pancreatic beta cells and insulin secretion and subsequent changes in blood glucose levels in STZ-induced type I diabetic models. In our study, beta cells were destructed by STZ, which makes the cells less active leading to poor sensitivity of insulin for glucose uptake by tissues and results in chronic hyperglycemia (20). In type II diabetes, beta cells are intact and therefore troxerutin may facilitate insulin secretion and/or its signaling leading to uptake of glucose from the blood; this represents the most important process to regulate glucose homeostasis (23). Evidence supporting this hypothesis is that in type II diabetes, administration of troxerutin decreased the blood glucose level as a result of improved insulin sensitivity and activity of insulin signaling molecules (22, 23). However, further studies are needed to get a precise and clear conclusion in this regard and about the underlying mechanisms related to insulin-sensitizing effect of troxerutin.
Although, troxerutin is used in the treatment of varicose veins and chronic venous insufficiency (19), its precise effects and mechanisms on vascular complications have not been investigated thoroughly in both normal and diabetic conditions. In this study, we found the development of vascular structural and pathological abnormalities in diabetic rat aorta. Nevertheless, troxerutin significantly reduced endothelial denudation and lipid deposition both in intimae and media of diabetic aorta, inhibited infiltration of inflammatory cells, appearance of tissue pyknotic nuclei and intercellular swelling and edema, and decreased deformity of vascular SMCs in the aorta of STZ-induced diabetic rats. It has been reported that troxerutin can improve vascular physiology through limiting abnormal leakage of varicose veins and attachment of platelets in endothelial cells (15, 19), as well as restoring diabetes-induced alterations of capillary structure in retinal vasculature, along with normalizing the expression levels of vascular endothelial growth factor (28). These findings suggest that treatment with troxerutin restores vascular structural and functional integrity and histopathological alterations in diabetic and other pathological conditions.
Development of oxidative stress is one of the major events for diabetic complications of vascular system and hyperglycemia has been suggested to be a key player in mediation of this oxidative stress effect on impairment of vascular integrity (3, 7). In mechanistic insight onto troxerutin effects, we showed that troxerutin reduced diabetes-associated marked increase in aortic lipid peroxidation marker, TBARS (assayed by MDA) in diabetic aorta, demonstrating the antioxidative activity of this agent in diabetes. In agreement with these results, troxerutin significantly improved hepatic lipid homeostasis (24), suppressed heart lipid abnormalities (22), ameliorated nickel-induced oxidative stress in plasma (29) and protected D-galactose-induced kidney, liver and brain injuries (15-18); all of them through its specific antioxidative potential and inhibiting the oxidative stress markers. Even though, we could not measure direct generation of ROS and oxidant anions such as O2- or OH- radicals and this is one of the limitations of this study, instead, we assessed aortic levels of endogenous antioxidants SOD and GPX, which indirectly indicate the oxidation status. As mentioned above, MDA-lowering effect of troxerutin was associated with its influence on GPX level in diabetic aorta, supporting its antioxidation mechanism. However, the levels of antioxidant enzymes remained approximately unchanged in diabetic rats compared to controls; this may be due to compensatory increase in the activity of defensive system in response to overproduction of ROS induced by hyperglycemia. Nevertheless, increased levels of MDA along with unchanged levels of enzymes suggest the imbalance between ROS generation and defensive antioxidation system leading to peroxidative injury in diabetic aorta compared to controls.
Lipids in the plasma membranes of cells, mitochondria and endoplasmic reticulum as well as proteins and nucleotide acids inside the cells are the main targets of ROS to generate peroxidation end products, which can be toxic to cells (4, 30). Superoxide anion is a primary precursor of ROS and can be converted to H2O2 by SOD (4). This enzyme along with GPX could reverse the endothelial dysfunction of diabetic artery. GPX converts H2O2 to water and lipid peroxides to their corresponding alcohols (4, 7). Detoxification of secondary oxidation products is vital and GPX plays an important role in reducing lipid peroxides (4). However, some previous studies demonstrated that SOD was ineffective (6, 31) and inhibition of hydroxyl radical formation would be more potent that SOD in mediation of aortic endothelial dysfunction in STZ-induced diabetic rats (32); these discrepancies might be attributed to differences in species and mechanisms of dysfunction in various vascular beds or variation in experimental conditions (6).
It is believed that lowering the blood glucose would inhibit the ROS injury and related vascular complications in diabetes (3, 4, 30). However, beneficial effects of troxerutin on aortic tissue were not associated with its effect on hyperglycemia in our model of STZ-induced diabetic rats; therefore, these are likely due to the effects other than a hypoglycemic mechanism. To support this finding, restoration of normoglycemia could not reduce upregulation of free radical generation and vascular dysfunction in diabetic mice and human endothelial cells (3, 33). Indeed, hyperglycemia-induced tissue damages were shown to be mediated via several mechanisms, including increased formation of advanced glycation end-product (AGE), activation of PKC isoforms, endoplasmic reticulum stress, mitochondrial dysfunction, apoptosis, as well as ROS formation (7, 30, 34). One of the main causes of ROS production and thereby structural and functional changes in vasculature in the setting of hyperglycemia is activation of PKC and its downstream targets such as NF-κB and vascular adhesion molecules (30-33). We did not investigate the mechanisms of troxerutin-induced reduction in oxidative stress, but it can be assumed that troxerutin may inhibit over-activation of PKC or its targets in diabetic aorta. Along these lines, troxerutin has been shown to reduce the production of AGEs, block the endoplasmic reticulum stress pathway and inhibit the activation of inflammatory pathway JNK1/IKKb/NF-κB in the hippocampus of mice fed a high-cholesterol diet (14, 18). On the other hand, inflammation is one of the mechanisms often associated with oxidative stress (15, 35). Troxerutin has also blocked D-galactose-induced increase in the levels of inflammatory markers p65, COX2 and iNOS in renal and hepatic tissues of diabetic rats (15, 16). Therefore, one may attribute the beneficial effects of troxerutin observed in this study to its multimechanistic actions that are not directly related to its hypoglycemic or SOD-stimulating activities (16, 23).
Study limitations: we could not perform direct measurement of ROS and oxidant anions generation instead of antioxidative enzymes, did not investigate the mechanisms of troxerutin-induced reduction in oxidative stress and did not evaluate the expression levels of glucose transporters in rat aorta. The results of all these might help us to better understanding and clear elucidation of troxerutin effects on diabetic aorta. Therefore, these remarks would be suggested as the targets of future investigations.
In conclusion, our study suggested that troxerutin has a therapeutic potential in preventing diabetic vascular abnormalities and pathological damages in rat aorta, which might be mediated partly through reduction in lipid peroxidation and improvement in endogenous antioxidative activity and subsequent inhibition of oxidative stress.
Footnotes
Authors’ Contributions:Reza Badalzadeh: Study concept and design, study supervision and drafting of the manuscript; Nayeleh Layeghzadeh: acquisition of data and drafting of the manuscript; Alireza Alihemmati: analysis and interpretation of data and statistical analysis; Mustafa Mohammadi: critical revision of the manuscript for important intellectual content.
Funding/Support:This work was derived from Nayeleh Layeghzadash thesis (92.1-3.5) and supported by Drug Applied Research Center, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care. 2011;34 Suppl 2:S285–90. doi: 10.2337/dc11-s239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34(31):2436–43. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sena CM, Pereira AM, Seica R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832(12):2216–31. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Fowler MJ. Microvascular and Macrovascular Complications of Diabetes. Clin Diabetes. 2008;26(2):77–82. [Google Scholar]
- 6.Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Markers of Oxidative Stress during Diabetes Mellitus. J Biom. 2013;2013:8. doi: 10.1155/2013/378790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19(32):5695–703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- 8.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alper G, Olukman M, Irer S, Caglayan O, Duman E, Yilmaz C, et al. Effect of vitamin E and C supplementation combined with oral antidiabetic therapy on the endothelial dysfunction in the neonatally streptozotocin injected diabetic rat. Diabetes Metab Res Rev. 2006;22(3):190–7. doi: 10.1002/dmrr.586. [DOI] [PubMed] [Google Scholar]
- 10.Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59(7):365–73. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Wang F, Hu S. The electrochemical oxidation of troxerutin and its sensitive determination in pharmaceutical dosage forms at PVP modified carbon paste electrode. Colloids Surf B Biointerfaces. 2006;52(1):8–13. doi: 10.1016/j.colsurfb.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Heidarzadeh F, Badalzadeh R, Hatami H. The effect of troxerutin on lipid peroxidation and tissue injury induced by myocardial ischemia reperfusion injury in diabetic rat. Razi J Med Sci. 2014;21(125):37–45. [Google Scholar]
- 13.Maurya DK, Balakrishnan S, Salvi VP, Nair CK. Protection of cellular DNA from gamma-radiation-induced damages and enhancement in DNA repair by troxerutin. Mol Cell Biochem. 2005;280(1-2):57–68. doi: 10.1007/s11010-005-8052-3. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Wu DM, Zheng YL, Hu B, Cheng W, Zhang ZF, et al. Troxerutin counteracts domoic acid-induced memory deficits in mice by inhibiting CCAAT/enhancer binding protein beta-mediated inflammatory response and oxidative stress. J Immunol. 2013;190(7):3466–79. doi: 10.4049/jimmunol.1202862. [DOI] [PubMed] [Google Scholar]
- 15.Fan SH, Zhang ZF, Zheng YL, Lu J, Wu DM, Shan Q, et al. Troxerutin protects the mouse kidney from d-galactose-caused injury through anti-inflammation and anti-oxidation. Int Immunopharmacol. 2009;9(1):91–6. doi: 10.1016/j.intimp.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q, et al. Troxerutin protects the mouse liver against oxidative stress-mediated injury induced by D-galactose. J Agric Food Chem. 2009;57(17):7731–6. doi: 10.1021/jf9012357. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Wu DM, Hu B, Cheng W, Zheng YL, Zhang ZF, et al. Chronic administration of troxerutin protects mouse brain against D-galactose-induced impairment of cholinergic system. Neurobiol Learn Mem. 2010;93(2):157–64. doi: 10.1016/j.nlm.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Wu DM, Zheng ZH, Zheng YL, Hu B, Zhang ZF. Troxerutin protects against high cholesterol-induced cognitive deficits in mice. Brain. 2011;134(Pt 3):783–97. doi: 10.1093/brain/awq376. [DOI] [PubMed] [Google Scholar]
- 19.Gohel MS, Davies AH. Pharmacological agents in the treatment of venous disease: an update of the available evidence. Curr Vasc Pharmacol. 2009;7(3):303–8. doi: 10.2174/157016109788340758. [DOI] [PubMed] [Google Scholar]
- 20.Pari L, Monisha P, Mohamed Jalaludeen A. Beneficial role of diosgenin on oxidative stress in aorta of streptozotocin induced diabetic rats. Eur J Pharmacol. 2012;691(1-3):143–50. doi: 10.1016/j.ejphar.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 21.Badalzadeh R, Mohammadi M, Najafi M, Ahmadiasl N, Farajnia S, Ebrahimi H. The additive effects of ischemic postconditioning and cyclosporine-A on nitric oxide activity and functions of diabetic myocardium injured by ischemia/reperfusion. J Cardiovasc Pharmacol Ther. 2012;17(2):181–9. doi: 10.1177/1074248411416118. [DOI] [PubMed] [Google Scholar]
- 22.Geetha R, Yogalakshmi B, Sreeja S, Bhavani K, Anuradha CV. Troxerutin suppresses lipid abnormalities in the heart of high-fat-high-fructose diet-fed mice. Mol Cell Biochem. 2014;387(1-2):123–34. doi: 10.1007/s11010-013-1877-2. [DOI] [PubMed] [Google Scholar]
- 23.Sampath S, Karundevi B. Effect of troxerutin on insulin signaling molecules in the gastrocnemius muscle of high fat and sucrose-induced type-2 diabetic adult male rat. Mol Cell Biochem. 2014;395(1-2):11–27. doi: 10.1007/s11010-014-2107-2. [DOI] [PubMed] [Google Scholar]
- 24.Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q, et al. Troxerutin improves hepatic lipid homeostasis by restoring NAD(+)-depletion-mediated dysfunction of lipin 1 signaling in high-fat diet-treated mice. Biochem Pharmacol. 2014;91(1):74–86. doi: 10.1016/j.bcp.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Fraga CG, Leibovitz BE, Tappel AL. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med. 1988;4(3):155–61. doi: 10.1016/0891-5849(88)90023-8. [DOI] [PubMed] [Google Scholar]
- 26.Badalzadeh R, Mohammadi M, Yousefi B, Faranjia S, Najafi M, Mohammadi S. Involvement of Glycogen Synthase Kinase-3β and Oxidation Status in the Loss of Cardioprotection by Postconditioning in Chronic Diabetic Male Rats. Adv Pharm Bull. 2015;5(1) doi: 10.15171/apb.2015.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghyasi R, Sepehri G, Mohammadi M, Badalzadeh R, Ghyasi A. Effect of mebudipine on oxidative stress and lipid peroxidation in myocardial ischemic-reperfusion injury in male rat. J Res Med Sci. 2012;17(12):1150–5. [PMC free article] [PubMed] [Google Scholar]
- 28.Chung HK, Choi SM, Ahn BO, Kwak HH, Kim JH, Kim WB. Efficacy of troxerutin on streptozotocin-induced rat model in the early stage of diabetic retinopathy. Arzneimittelforschung. 2005;55(10):573–80. doi: 10.1055/s-0031-1296907. [DOI] [PubMed] [Google Scholar]
- 29.Elangovan P, Pari L. Ameliorating effects of troxerutin on nickel-induced oxidative stress in rats. Redox Rep. 2013;18(6):224–32. doi: 10.1179/1351000213Y.0000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roghani Dehkordi F, Roghani M. Mechanisms Underlying Sesamolin-Induced Attenuation of Vascular Dysfunction in Rats With Streptozotocin-Induced Diabetes. Int J Endocrinol Metab. 2011;9(2):311–6. [Google Scholar]
- 31.Yan LJ. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J Diabetes Res. 2014;2014:137919. doi: 10.1155/2014/137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nassar T, Kadery B, Lotan C, Da'as N, Kleinman Y, Haj-Yehia A. Effects of the superoxide dismutase-mimetic compound tempol on endothelial dysfunction in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2002;436(1-2):111–8. doi: 10.1016/s0014-2999(01)01566-7. [DOI] [PubMed] [Google Scholar]
- 33.Paneni F, Mocharla P, Akhmedov A, Costantino S, Osto E, Volpe M, et al. Gene silencing of the mitochondrial adaptor p66(Shc) suppresses vascular hyperglycemic memory in diabetes. Circ Res. 2012;111(3):278–89. doi: 10.1161/CIRCRESAHA.112.266593. [DOI] [PubMed] [Google Scholar]
- 34.Patel H, Chen J, Das KC, Kavdia M. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc Diabetol. 2013;12:142. doi: 10.1186/1475-2840-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebrahimi H, Badalzadeh R, Mohammadi M, Yousefi B. Diosgenin attenuates inflammatory response induced by myocardial reperfusion injury: role of mitochondrial ATP-sensitive potassium channels. J Physiol Biochem. 2014;70(2):425–32. doi: 10.1007/s13105-014-0320-9. [DOI] [PubMed] [Google Scholar]