Abstract
Background and objectives
Although several standardized definitions for AKI have been developed, no consensus exists regarding which to use in children. This study applied the Pediatric RIFLE (pRIFLE), AKI Network (AKIN), and Kidney Disease Improving Global Outcomes (KDIGO) criteria to an anonymized cohort of hospitalizations extracted from the electronic medical record to compare AKI incidence and outcomes in intensive care unit (ICU) and non-ICU pediatric populations.
Design, setting, participants, & measurements
Observational, electronic medical record–enabled study of 14,795 hospitalizations at the Lucile Packard Children’s Hospital between 2006 and 2010. AKI and AKI severity stage were defined by the pRIFLE, AKIN, and KDIGO definitions according to creatinine change criteria; urine output criteria were not used. The incidences of AKI and each AKI stage were calculated for each classification system. All-cause, in-hospital mortality and total hospital length of stay (LOS) were compared at each subsequent AKI stage by Fisher exact and Kolmogorov–Smirnov tests, respectively.
Results
AKI incidences across the cohort according to pRIFLE, AKIN, and KDIGO were 51.1%, 37.3%, and 40.3%. Mortality was higher among patients with AKI across all definitions (pRIFLE, 2.3%; AKIN, 2.7%; KDIGO, 2.5%; P<0.001 versus no AKI [0.8%–1.0%]). Within the ICU, pRIFLE, AKIN, and KDIGO demonstrated progressively higher mortality at each AKI severity stage; AKI was not associated with mortality outside the ICU by any definition. Both in and outside the ICU, AKI was associated with significantly higher LOS at each AKI severity stage across all three definitions (P<0.001). Definitions resulted in differences in diagnosis and staging of AKI; staging agreement ranged from 76.7% to 92.5%.
Conclusions
Application of the three definitions led to differences in AKI incidence and staging. AKI was associated with greater mortality and LOS in the ICU and greater LOS outside the ICU. All three definitions demonstrated excellent interstage discrimination. While each definition offers advantages, these results underscore the need to adopt a single, universal AKI definition.
Keywords: acute renal failure, children, kidney failure, mortality
Introduction
Acute renal failure and AKI have traditionally been used to describe an abrupt decline in renal function; until recently, however, a standardized definition for this condition did not exist. Recognizing that the lack of a consistent definition hindered research efforts, the Acute Dialysis Quality Initiative group created the RIFLE criteria in 2004, establishing a multidimensional, staged definition (1). Since then, the RIFLE criteria have been modified three times. The first modification, the Pediatric RIFLE (pRIFLE) criteria, adapted the RIFLE criteria for use in children (2). The second modified definition, the AKI Network (AKIN) criteria, expanded the diagnosis of AKI to include patients who experienced a ≥0.3-mg/dl increase in serum creatinine in a 48-hour period (3). The most recent modification, the Kidney Disease Improving Global Outcomes (KDIGO) classification system, harmonized RIFLE, AKIN, and pRIFLE (4).
While each new definition has offered refinement, no definition has been shown to be superior and no universal consensus exists as to which definition to use (5,6). For example, in 2013 alone, studies using each of the three definitions were published. Furthermore, a recent study proposed the use of an entirely different AKI definition based on absolute, rather than relative, creatinine changes (7). Taken together, this is a potentially troubling trend away from establishing a unified AKI diagnosis.
The ability to accurately and consistently identify AKI is of paramount importance in pediatric inpatients. AKI is common, occurring in one third of hospitalized children (8). In addition, AKI has been associated with higher morbidity and mortality, both in critical care and non–critical care pediatric populations (9–14). Inconsistent definition of AKI will lead to erratic diagnosis and staging, hindering our ability to improve outcomes and making it more challenging to treat and prevent AKI episodes.
The objective of this study was to compare AKI incidence as well as staged mortality and length of stay (LOS) information among the three AKI definitions currently used in children. Our ultimate goal is to integrate an automated AKI diagnostic tool into the electronic medical record (EMR); the application of the three definitions to a large cohort of pediatric hospitalizations created by extracting anonymized EMR data is the first step toward that goal.
Materials and Methods
Study Design
We conducted a retrospective, EMR-enabled study at Lucile Packard Children’s Hospital (Stanford University, Stanford, CA). All pediatric (<18 years) hospitalizations occurring between January 1, 2006, and December 31, 2010, with at least one serum creatinine obtained during that time were considered for inclusion. Data were extracted anonymously without distinguishing identifiers (age in days at admission; transfer; discharge; all serum creatinine values obtained between January 1, 2006, and December 31, 2010; sex; height; patient location; and in-hospital mortality). Because neonatal AKI represents a unique spectrum of disease and neonatal renal physiology makes application of standard AKI definitions challenging (15,16), admissions to neonatal care wards were excluded. Additional exclusions included patients without sex documentation, baseline creatinine, and follow-up creatinine values. Baseline creatinine was defined as the most recent creatinine obtained in the 3 months before admission, inclusive of the day of admission. Because the study used de-identified patient data, it was exempt from institutional review board review.
Definition of AKI
AKI was defined using three sets of criteria (Table 1): pRIFLE (2), AKIN (3), and KDIGO (4). For each definition, only the creatinine criteria were used; urine output criteria were not applied. In addition, given the automated data extraction method used, it was not possible to obtain data on RRT use; thus, the RRT provision for stage 3 was not used. pRIFLE subdivides AKI into three severity stages (risk, injury, and failure) and two outcomes (loss and ESRD), whereas both AKIN and KDIGO use only three severity stages (stage 1, stage 2, and stage 3). Operationally, stage 1 corresponds to risk, stage 2 corresponds to injury, and stage 3 corresponds to failure. For the sake of clarity, we used stage 1, 2, and 3 across all definitions. Stage-specific criteria and operational modifications to each definition are shown in Table 1. eGFR was estimated using the Schwartz method (17); creatinine determinations were performed using the Jaffe technique. Because children younger than 3 months of age have rapidly changing GFRs, we applied only the eGFR<35 ml/min per 1.73 m2 criteria (KDIGO and pRIFLE stage 3) to children older than 3 months of age.
Table 1.
Staged diagnostic criteria for AKI
| Definition and Criteria for AKI Stages | Modifications |
|---|---|
| pRIFLE | |
| Stage 1 (Risk): eGFR decreased by 25% | |
| Stage 2 (Injury): eGFR decreased by 50% | |
| Stage 3 (Failure): eGFR decrease by 75% | |
| or | |
| eGFR <35 ml/min per 1.73 m2 | |
| AKIN | |
| Stage 1: Increase in creatinine of ≥50% | 0.3-mg/dl increase added to stage 1 |
| or | AKI diagnosed over 48-hr period |
| Absolute increase in creatinine of 0.3 mg/dl | |
| Stage 2: Increase in creatinine of ≥100% | |
| Stage 3: Increase in creatinine of ≥200% | |
| KDIGO | |
| Stage 1: Increase in creatinine of ≥50% | eGFR threshold from pRIFLE added to stage 3 |
| or | Creatinine changes (except absolute 0.3-mg/dl increase) required to occur within a 7-d time frame |
| Absolute increase in creatinine of 0.3 mg/dl | |
| Stage 2: Increase in creatinine of ≥100% | |
| Stage 3: Increase in creatinine of ≥200% | |
| or | |
| eGFR ≤35 ml/min per 1.73 m2 (if age <18 yr) |
eGFR was estimated using the Schwartz method. pRIFLE, pediatric RIFLE; AKIN, Acute Kidney Injury Network; KDIGO, Kidney Diseases Improving Global Outcomes.
Statistical Analyses
Analyses were performed at the hospitalization level, and the primary independent variable was AKI or AKI stage. The primary outcomes of interest were all-cause, in-hospital mortality and total hospital LOS. Where heights were not obtained, they were extrapolated by assuming constant growth rates (cm/day) over each 1-day period for patients younger than 10 days of age, each 10-day period for patients age 10–99 days, and each 100-day period for patients between 100 days and 18 years of age. Growth rates were estimated from all heights measured across the entire population. Given these rates, patient height on a given date was estimated on the basis of the closest available height measurement; for patients without any heights available, the average height across the population for that age was imputed. Within the three definitions, the mortality for each AKI stage was compared with the mortality in patients without AKI, as well as the mortality in patients with the preceding AKI severity stage (stage n versus stage n−1, where stage 0 was no AKI). P values were determined using a Fisher exact test.
In addition, mortality likelihood ratios were calculated for each AKI stage within all three definitions, comparing the likelihood of death within that stage to the likelihood of death among patients without AKI. LOS data for each AKI stage within each definition were assessed using the Kolmogorov–Smirnov test. As with mortality, LOS for each AKI severity stage was compared with that of the stage immediately preceding it, as well as with that of patients without AKI. We applied the Bonferroni correction to all comparative tests examining mortality and LOS. As we ran 27 tests (three stages for three definitions on three populations), we report P<0.05 only when our individual tests reported P<0.05/27; we report P<0.001 only when our individual tests reported P<0.001/27. We reported medians (interquartile range) and means±SD for non-normally and normally distributed data, respectively. All analyses were performed with the SciPy package (www.scipy.org) and Python (www.python.org).
Results
Cohort Demographic Characteristics
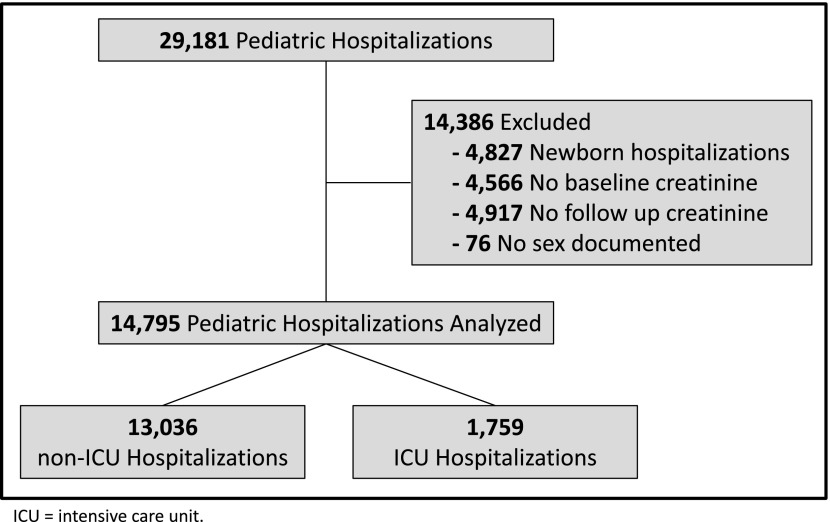
We identified 29,181 hospitalizations across which 188,032 serum creatinine values were obtained. After exclusion of 4827 visits to newborn care units, 4566 visits without a baseline creatinine, 4917 visits without a follow-up creatinine, and 76 visits without a documented sex, 14,795 hospitalizations remained (Figure 1). Excluded patients were younger (median age, 5.0 versus 6.7 years; P<0.001), had shorter LOS (median, 2 versus 5 days; P<0.001), and had lower mortality (mortality, 0.53% versus 1.6%; P<0.001). For the included hospitalizations, the median age was 6.70 years (IQR, 1.95–14.11 years) and heights were available for 71% (66% of ICU hospitalizations and 71% of non-ICU hospitalizations). Of the 14,795 hospitalizations, 1759 were classified as ICU (median age, 6.57 years; IQR, 2.10–14.73 years) and 13,036 were classified as non-ICU (median age, 6.71; IQR, 1.93–14.02 years).
Figure 1.
Cohort creation, exclusion criteria, and subgroup definition. ICU, intensive care unit.
AKI Incidence
AKI incidences according to pRIFLE, AKIN, and KDIGO were 51.1%, 37.3%, and 40.3%, respectively. The largest disparities were found in stages 1 and 3 (Figure 2). The incidences of stage 1 AKI according to pRIFLE, AKIN, and KDIGO were 26.9%, 19.4%, and 18.2%, respectively. The incidences of stage 3 AKI according to pRIFLE, AKIN, and KDIGO were 10.8%, 6.7%, and 11.7%, respectively. The incidences of AKI in the ICU and non-ICU populations were similar across all three definitions: pRIFLE, 51.3% for ICU and 51.0% for non-ICU; AKIN, 39.9% for ICU and 37.6% for non-ICU; KDIGO, 42.0% for ICU and 40.5% for non-ICU).
Figure 2.
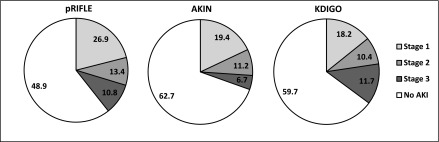
Incidence of AKI according to three definitions. The incidence for each AKI stage is shown as a percentage. AKIN, AKI Network; KDIGO, Kidney Diseases Improving Global Outcomes; pRIFLE, pediatric RIFLE.
AKI Stage and Mortality
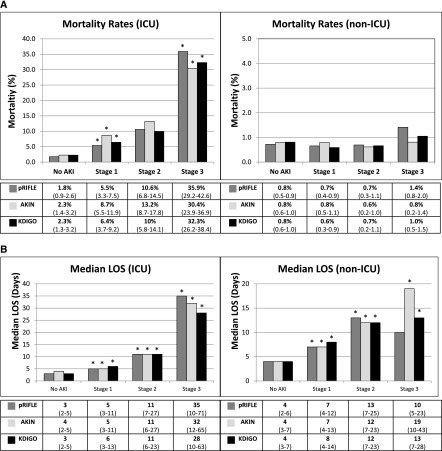
Across the cohort, all-cause, in-hospital mortality was 1.6%. Mortality was higher among hospitalizations with AKI than those without across all definitions (pRIFLE, 2.3% versus 0.8%; AKIN, 2.7% versus 1.0%; KDIGO, 2.5% versus 1.0%; P<0.001). Within the ICU, hospitalizations with AKI had significantly higher mortality than those without (pRIFLE, 13.4% versus 1.8%; AKIN, 16.0% versus 2.3%; KDIGO, 15.3% versus 2.3%; all P<0.001). This was true regardless of AKI severity stage; across all definitions, patients with stages 1, 2, and 3 AKI had higher mortality than patients who did not experience AKI (Table 2) (P<0.001). Additionally, within the ICU, increasing AKI severity was associated with progressively higher mortality (Figure 3A); compared with the preceding AKI severity stage, mortality was significantly higher for stages 1 and 3 across all three definitions (P<0.05). In the non-ICU hospitalizations, AKI was not associated with significantly greater mortality (pRIFLE, 0.7% versus 0.8%; AKIN, 0.8% versus 0.7%; KDIGO, 0.8% versus 0.7%).
Table 2.
Mortality likelihood ratios for AKI severity stages
| Definition | All Hospitalizations | ICU | Non-ICU | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 1 | Stage 2 | Stage 3 | Stage 1 | Stage 2 | Stage 3 | |
| pRIFLE | 1.44 (0.99 to 2.09) | 2.28 (1.53 to 3.41) | 6.64 (4.82 to 9.15) | 3.08 (1.64 to 5.79) | 6.06 (3.26 to 11.3) | 20.49 (12.0 to 35.0) | 0.91 (0.55 to 1.50) | 0.96 (0.51 to 1.81) | 1.96 (1.17 to 3.31) |
| AKIN | 1.69 (1.19 to 2.41) | 2.29 (1.57 to 3.35) | 6.38 | 3.83 | 5.82 | 13.37 | 1.0 | 0.77 | 1.02 |
| KDIGO | 1.30 (0.87 to 1.93) | 1.90 (1.25 to 2.89)a | 5.15 (3.84 to 6.90)a | 2.85 (1.59 to 5.13)a | 4.42 (2.47 to 7.89)a | 14.34 (9.18 to 22.4)a | 0.73 (0.41 to 1.30) | 0.82 (0.41 to 1.64) | 1.22 (0.69 to 2.13) |
The likelihood of death (and 95% confidence interval) for each AKI severity stage is compared with that of hospitalizations not complicated by AKI. ICU, intensive care unit.
P<0.05 after correction for multiple testing.
Figure 3.
Mortality and hospital length of stay (LOS) by AKI severity stage in ICU and non-ICU hospitalizations. (A) Mortality rates for patients with no AKI, stage 1 AKI, stage 2 AKI, and stage 3 AKI are presented as percentages with 95% confidence intervals. Mortality was compared with that of the previous stage. *P<0.05. Within the ICU, mortality was significantly higher for pRIFLE stages 1, 2, and 3; for AKIN stages 1 and 3; and for KDIGO stages 1 and 3 (P<0.05 for all). Outside of the ICU, higher AKI severity stage was not associated with higher mortality. (B) The total hospital LOS data for patients with no AKI, stage 1 AKI, stage 2 AKI, and stage 3 AKI are presented as medians (interquartile range). LOS was compared with that in the preceding stage. *P<0.05. Within the ICU, LOS was progressively higher at all three stages across all three definitions (P<0.001). Outside the ICU, LOS was progressively higher at all three stages across all three definitions except for pRIFLE stage 3 (P<0.001).
AKI Stage and LOS
Median LOS was higher among hospitalizations with AKI than those without across all definitions (pRIFLE, 9 versus 4 days; AKIN, 10 versus 4 days; KDIGO, 10 versus 4 days; P<0.001). According to pRIFLE, median LOS was 7 days (IQR, 4–12 days) for stage 1, 13 days (IQR, 7–25 days) for stage 2, and 11 days (IQR, 5–27 days) for stage 3 (P<0.001 versus no-AKI and versus preceding stage). LOS was higher when the AKIN (stage 1, 7 days [IQR, 4–13 days]; stage 2, 12 days [IQR, 7–23 days]; stage 3, 20 days [IQR, 10–44 days]) and KDIGO (stage 1, 8 days [IQR, 4–14 days]; stage 2, 12 days [IQR, 7–23 days]; stage 3, 13 days [IQR, 7–30 days]) definitions were applied (P<0.001 versus no-AKI and versus preceding stage). When the hospitalizations were split into ICU and non-ICU hospitalizations, greater AKI severity stage continued to be associated with longer LOS in both settings across all three definitions (P<0.001 versus no-AKI and versus preceding stage) (Figure 3B). The one exception was pRIFLE stage 3; while these hospitalizations had a higher LOS than those without AKI, they also had a slightly shorter LOS than hospitalizations with stage 2 AKI.
Interdefinition Agreement
Application of the three definitions did not lead to similar diagnosis or staging of patients. Regarding the diagnosis of AKI, AKIN agreed with pRIFLE 84.5% (κ=0.69) of the time, KDIGO agreed with pRIFLE 87.3% (κ=0.75) of the time, and AKIN agreed with KDIGO 97.1% (κ=0.94) of the time. Additionally, patients with AKI were staged differently on the basis of the definition applied. AKIN and pRIFLE agreed on AKI stage 76.7% (κ=0.63) of the time, KDIGO and pRIFLE agreed on AKI stage 84.2% (κ=0.75) of the time, and AKIN and KDIGO agreed upon AKI stage 92.5% (κ=0.87) of the time.
AKI overlap across the three definitions is shown in Figure 4. pRIFLE identified the most AKI cases and AKIN identified the fewest. A total of 1720 patients were diagnosed with AKI just by pRIFLE. By contrast, AKIN and KDIGO identified zero and six such patients, respectively. The patients classified as having AKI only by pRIFLE had lower mortality than patients classified as having AKI by all three definitions (1.5% versus 2.7%; P<0.001). pRIFLE failed to identify 153 patients classified as having AKI by AKIN and KDIGO, whereas AKIN failed to identify 427 patients classified as having AKI by pRIFLE and KDIGO. No patients were diagnosed by AKIN and pRIFLE but not KDIGO. In patients whom pRIFLE and AKIN failed to classify as having AKI, mortality was similar to that in patients without AKI by any definition (0% versus 0.7% versus 0.8%).
Figure 4.
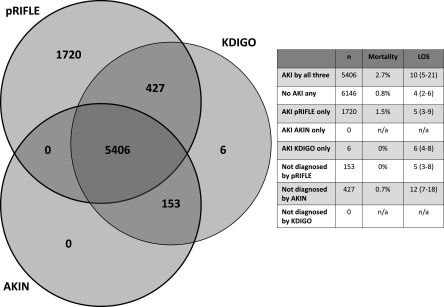
Definitional overlap and outcomes for specific patient cohorts. The diagram demonstrates how many patients were diagnosed with AKI according to each of the three definitions. For example, 5406 patients were diagnosed by all three definitions, zero were diagnosed by AKIN and pRIFLE but not by KDIGO, 1720 were diagnosed by pRIFLE alone, and 427 were diagnosed by pRIFLE and KDIGO but not by AKIN. Data on mortality and LOS for patients not identified by each definition and diagnosed solely by each definition are also presented. n/a, not applicable.
Discussion
AKI is common among hospitalized children. Depending on the definition used, AKI occurred in 37%–51% of at-risk hospitalizations; at-risk was defined as having a baseline and at least one additional creatinine obtained. This is consistent with previously published reports using newer, standard definitions based on creatinine change (8,13,18,19). Our study also suggests that much pediatric AKI is of mild severity as 45%–53% of cases were classified as stage 1. Nevertheless, AKI was significantly associated with greater mortality and higher LOS according to all three definitions.
Application of the three definitions resulted in different AKI incidences. This is similar to the findings of Zappitelli et al., who compared pRIFLE and AKIN among pediatric inpatients. They found pRIFLE more sensitive because it identified more stage 1 events (19). In our study, pRIFLE also created the largest stage 1 cohort. We also noted that while pRIFLE and KDIGO resulted in similarly sized stage 3 cohorts, AKIN detected the smallest stage 3 cohort; this is likely because AKIN bases stage 3 only on percentage change in creatinine, whereas pRIFLE and KDIGO also use an eGFR≤35 ml/min per 1.73 m2 criteria for stage 3. These staging discrepancies were highlighted by our comparative data; while AKIN and KDIGO agreed on staging 92.5% of the time, agreement between AKIN and pRIFLE was only 76.7%. These differences, both in overall incidence and staging, are troubling if the literature continues to apply multiple definitions. It may become difficult to compare results of studies that used different definitions, especially from an epidemiologic standpoint. This underscores the need for a single, unified AKI definition.
We did find that across all three definitions, AKI is associated with higher mortality; this is predominantly driven by the ICU population, in which death is more frequent. Within the ICU, all three definitions demonstrated higher mortality as severity stage increased. Outside the ICU, we found no association between AKI and mortality. However, this is more likely a product of the population, in which death is infrequent, than an indication of definitional weakness. This became apparent when LOS data were analyzed; greater AKI severity was associated with longer LOS both in and out of the ICU across all three definitions. Overall, these findings suggest that ICU AKI and non-ICU AKI represent different entities. Stage 2 AKI within the ICU is probably not the same as stage 2 AKI in patients who are not critically ill; they probably have different risks and are associated with different outcomes. This is consistent with the findings of Sutherland et al., who demonstrated that ICU AKI was associated with greater mortality (32.8% versus 9.4%) and longer LOS (29 days versus 6 days) than non-ICU AKI (9).
Although prior studies comparing definitions exist, ours is one of the largest to date and the first pediatric study to include both ICU and non-ICU populations. Furthermore, to our knowledge, our study is the first in children to demonstrate significantly higher mortality and LOS at each of the three AKI stages. Prior studies have yielded discrepant results. Bastin et al. compared the RIFLE, AKIN, and KDIGO definitions in 1881 adults undergoing cardiac surgery; while AKIN and KDIGO resulted in identical outcomes, both correlated better with mortality than did RIFLE (20). Sampaio et al. compared the three definitions in 321 patients undergoing cardiac surgery; in contrast, they found that KDIGO had superior prognostic power (21). Roy et al. compared the three definitions in 637 adults with heart failure and found a marginal difference in predictive ability (22). Zeng et al. found that the incidence of AKI varied when RIFLE, AKIN, and KDIGO were used; however, AKI by any definition was associated with higher mortality and cost (23). Using a technique similar to ours, Fujii et al. examined the three definitions across nearly 50,000 adult hospitalizations (24). They found that RIFLE and KDIGO resulted in identical incidences but that AKIN led to a lower incidence; additionally, RIFLE and KDIGO demonstrated better mortality discrimination than did AKIN. Lex et al. examined the pRIFLE, AKIN, and KDIGO definitions in children undergoing corrective cardiac surgery (25). They too found that as each of the three definitions was applied, patients transitioned between AKI stages; furthermore, the AKI cohorts defined by each set of criteria experienced different mortality.
In our study, differences among the definitions were apparent. pRIFLE created the largest AKI cohort, identifying the most stage 1 cases. AKIN was the most selective; its AKI diagnostic timeframe of 48 hours was the most restrictive, and the absence of the eGFR<35 ml/min per 1.73m2 criteria was likely responsible for a smaller stage 3 population. KDIGO resulted in an incidence that was between that of pRIFLE and AKIN. Notably, KDIGO is the only definition that contains both adult and pediatric criteria; the benefit of a harmonized definition is immense. However, both pRIFLE and KDIGO require patient heights (to calculate eGFR) for complete application of the definition; notably, no height was obtained for approximately 30% of our hospitalizations. While this may not be an issue in prospective studies, in retrospective studies the absence of height data is problematic and requires extrapolation (19). In addition, missing height data will hamper any attempt to integrate an AKI definition into an EMR to allow real-time detection or prediction.
Our findings should be interpreted in the context of their limitations. Our study is from a single, pediatric institution; the findings may not be generalizable to other centers or adult populations. We did not apply the urine output and RRT AKI criteria. However, the urine output criteria have never correlated with the creatinine criteria or outcomes, and it is unlikely that any patient classified as having stage 3 AKI by RRT criteria would not have done so by creatinine criteria. We also chose to include patients only if they had a baseline creatinine available and at least one follow-up creatinine during the hospitalization. We felt this to be appropriate given the pediatric population and our interest in future EMR integration. However, this choice probably had two effects. First, it likely biased the results toward the null. These excluded patients were less ill (lower mortality and LOS) and likely to have fallen in the no-AKI category were they included; more patients with lower mortality and shorter LOS in the no-AKI cohort would have increased the significance of the findings in the AKI cohort. Second, elimination of these hospitalizations probably overestimates the true incidence of AKI. However, the use of EMR-extracted data are a progressive step toward EMR integrated AKI identification, prediction, and prevention tools (26).
In summary, our findings demonstrate that pRIFLE, AKIN, and KDIGO result in different incidences and substantial disparities in staging. All three definitions correlate highly with outcomes and demonstrate excellent interstage discrimination. Greater AKI severity is associated with higher mortality and longer LOS in the ICU; it is associated with longer LOS in non–critically ill children. All three of the definitions offer advantages. pRIFLE is sensitive, identifying a greater number of mild AKI cases. AKIN does not require heights or baseline creatinine values; in EMR-related applications or retrospective research where height and baseline data are likely to be missing, this is advantageous. KDIGO offers applicability to both pediatric and adult populations and has a diagnostic timeframe that is less restrictive than AKIN. Regardless, our findings highlight the necessity of a unified AKI definition, and we recommend that the nephrology community continue to work toward this goal.
Disclosures
None.
Acknowledgments
We would like to acknowledge Gomathi Krishnan and Todd Ferris for their assistance with data acquisition. A portion of these data was accepted as an abstract for oral presentation at the 2013 American Society of Nephrology. It was presented on November 8, 2013 in Atlanta, Georgia.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup : Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL: Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71: 1028–1035, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury N, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.KDIGO AKI Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 5.Lafrance JP, Levin A: Defining AKI: Closer to getting the math right. Nephrol Dial Transplant 28: 1340–1342, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Valette X, du Cheyron D: A critical appraisal of the accuracy of the RIFLE and AKIN classifications in defining “acute kidney insufficiency” in critically ill patients. J Crit Care 28: 116–125, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Wang HE, Jain G, Glassock RJ, Warnock DG: Comparison of absolute serum creatinine changes versus Kidney Disease: Improving Global Outcomes consensus definitions for characterizing stages of acute kidney injury. Nephrol Dial Transplant 28: 1447–1454, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL, Acute Kidney Injury Advisory Group of the American Society of Nephrology : World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol 8: 1482–1493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, Ling XB: AKI in hospitalized children: Epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 8: 1661–1669, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL: 3-5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69: 184–189, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Blinder JJ, Goldstein SL, Lee VV, Baycroft A, Fraser CD, Nelson D, Jefferies JL: Congenital heart surgery in infants: Effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg 143: 368–374, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Goldstein SL, Devarajan P: Pediatrics: Acute kidney injury leads to pediatric patient mortality. Nat Rev Nephrol 6: 393–394, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Moffett BS, Goldstein SL: Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol 6: 856–863, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG: Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis 59: 523–530, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Ricci Z, Ronco C: Neonatal RIFLE. Nephrol Dial Transplant 28: 2211–2214, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Jetton JG, Askenazi DJ: Update on acute kidney injury in the neonate. Curr Opin Pediatr 24: 191–196, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 18.Zappitelli M, Moffett BS, Hyder A, Goldstein SL: Acute kidney injury in non-critically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: a retrospective cohort study. Nephrol Dial Transplant 26: 144–150, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL: Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol 3: 948–954, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastin AJ, Ostermann M, Slack AJ, Diller GP, Finney SJ, Evans TW: Acute kidney injury after cardiac surgery according to Risk/Injury/Failure/Loss/End-stage, Acute Kidney Injury Network, and Kidney Disease: Improving Global Outcomes classifications. J Crit Care 28: 389–396, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Sampaio MC, Máximo CA, Montenegro CM, Mota DM, Fernandes TR, Bianco AC, Amodeo C, Cordeiro AC: Comparison of diagnostic criteria for acute kidney injury in cardiac surgery. Arq Bras Cardiol 101: 18–25, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy AK, Mc Gorrian C, Treacy C, Kavanaugh E, Brennan A, Mahon NG, Murray PT: A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med 3: 26–37, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS: Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol 9: 12–20, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujii T, Uchino S, Takinami M, Bellomo R: Validation of the Kidney Disease Improving Global Outcomes criteria for AKI and comparison of three criteria in hospitalized patients. Clin J Am Soc Nephrol 9: 848–854, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lex DJ, Tóth R, Cserép Z, Alexander SI, Breuer T, Sápi E, Szatmári A, Székely E, Gál J, Székely A: A comparison of the systems for the identification of postoperative acute kidney injury in pediatric cardiac patients. Ann Thorac Surg 97: 202–210, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Colpaert K, Hoste E, Steurbaut K, Benoit D, Van Hoecke S, De Turck F, Decruyenaere J: Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class. Crit Care Med 40: 1164–1170, 2012 [DOI] [PubMed] [Google Scholar]