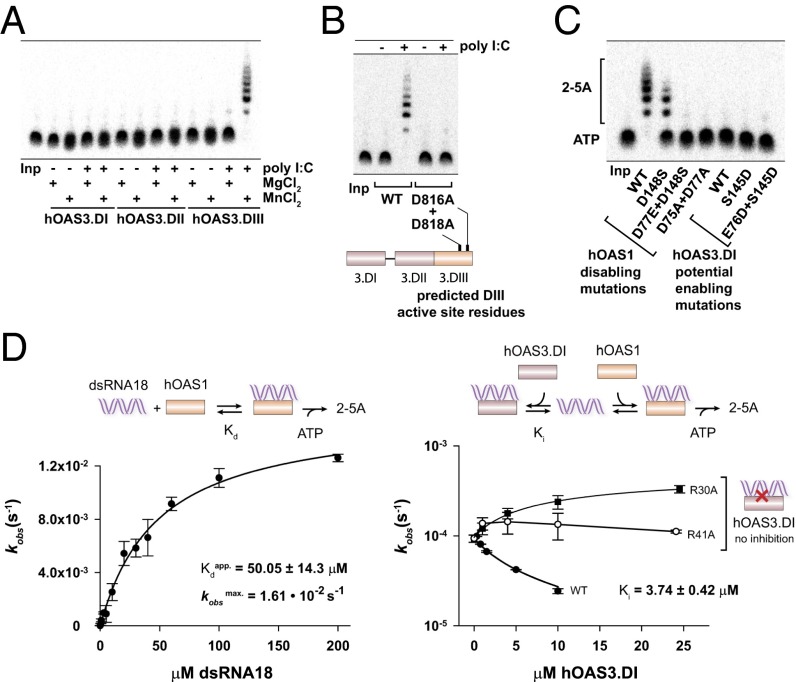
Fig. 3.
Roles of the individual domains of hOAS3 in sensing dsRNA. (A) Catalytic properties of individual domains of hOAS3 in the presence of dsRNA (2.5 OD260 poly I:C) and two divalent metal ions, Mg2+ or Mn2+. (B) Inactivation of hOAS3 by point mutations in the active site of domain DIII. Reactions contained full-length OAS3 (0.1 μM). (C) Active site mutagenesis in hOAS1 and hOAS3. Reactions contained 0.1 μM hOAS1 or hOAS3.DI, 2.5 OD260 poly I:C, 1 mM ATP and trace 32P-α-ATP, and were conducted at 37 °C for 2 h. Samples were resolved by 20% denaturing PAGE and visualized by phosphorimaging. (D, Left) activation of hOAS1 by dsRNA18 (Methods). (Right) Competition of hOAS3.DI and hOAS1 for binding to dsRNA18. WT hOAS3.DI (Ki = 3.74 μM) binds dsRNA stronger than hOAS1 (Kdapp = 50 μM), whereas two hOAS3.DI mutants that disrupt contacts to dsRNA (R30A and R41A) do not exhibit a detectable competition. Reactions contained 0.5 μM hOAS1 (Left), 1 μM hOAS1 (Right), and 100 nM dsRNA18 (Right). Data points show mean ± SD determined from at least two replicate time courses.