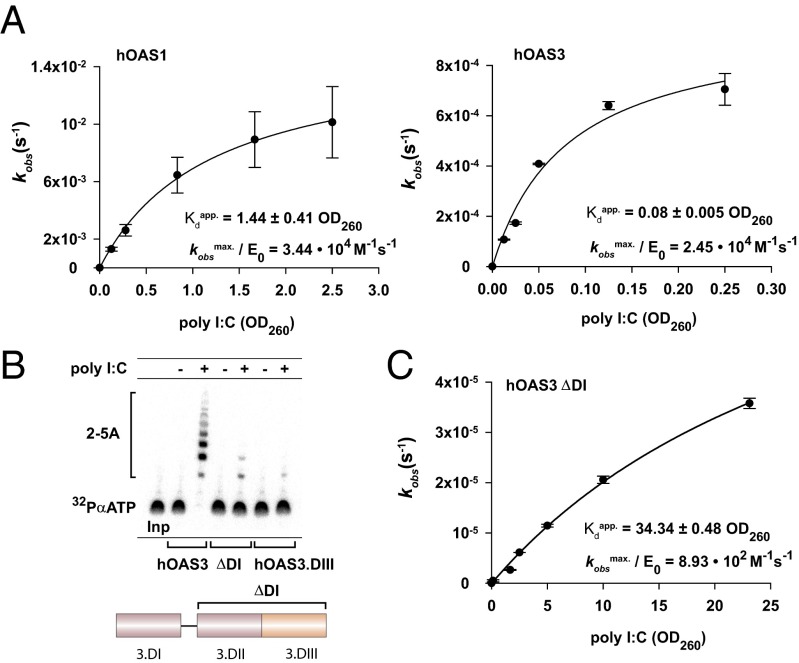
Fig. 4.
Importance of domain DI for the activation of hOAS3 by dsRNA. (A) Activation of hOAS1 (0.5 μM; Left) and hOAS3 (40 nM; Right) by poly I:C. (B) Comparison of 2-5A synthesis activity of hOAS3 full-length, isolated domains DII+DIII (ΔDI), and isolated domain DIII. Reactions contained 0.1 μM proteins and 2.5 OD260 poly I:C, as indicated, and were conducted at 37 °C for 2 h. (C) Dependence of the rate of 2-5A synthesis by ΔDI hOAS3 on poly I:C concentration. Reactions contained 0.1 μM ΔDI hOAS3. Apparent binding constants are specified in OD260 units. Data points represent mean ± SD determined from at least two replicate time courses. E0 is the total concentration of enzyme used.