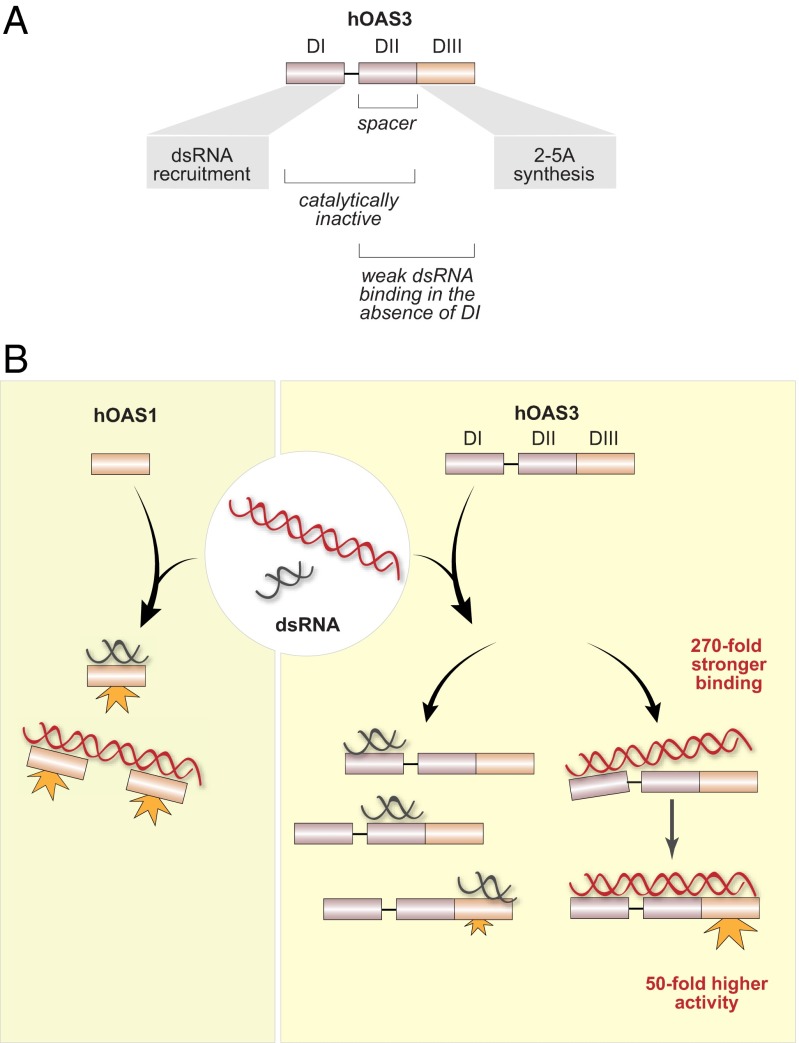
Fig. 6.
Model of dsRNA surveillance by hOAS1 and hOAS3. (A) Roles of the individual domains of hOAS3 in sensing dsRNA. (B) Human OAS1 recognizes long and short dsRNA indiscriminately. Multiple hOAS1 copies are allowed to bind to long dsRNA, however they are unlikely to functionally interact as hOAS1 exhibits similar specific activity with long dsRNA (>50 bp) and short dsRNA (<20 bp). In contrast, hOAS3 exhibits a strong (∼104-fold) preference for long dsRNA. Short dsRNA binds to the high-affinity domain DI, but not to the low-affinity domains DII/DIII, which is insufficient to activate hOAS3. Long dsRNA binds to the high-affinity domain DI and docks intramolecularly to the catalytically active domain DIII, activating hOAS3.