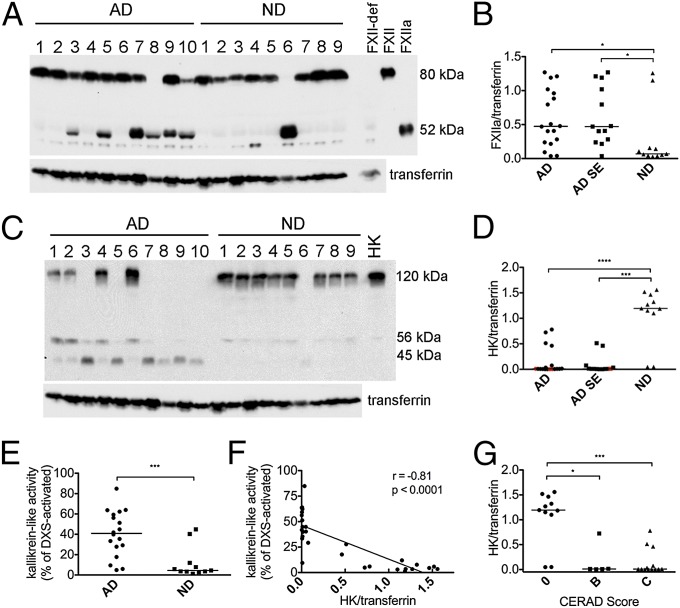
Fig. 1.
Activation of the FXIIa-driven contact system in AD patient plasma from group 1. (A) Western blot analysis of FXIIa and transferrin loading control in plasma of 18 AD patients and 11 ND controls from group 1 (representative samples shown), showing FXII zymogen (80 kDa) and the FXIIa heavy chain (52 kDa). Lane loaded with FXII-deficient human plasma (FXII-def) shows that the bands just below and above the FXIIa band are nonspecific. (B) FXIIa levels normalized to transferrin were significantly higher in AD (P = 0.029) than ND plasma. When AD cases with a history of stroke (n = 5) were excluded from the analysis (AD SE), FXIIa levels remained significantly higher than in ND plasma (P = 0.018). (C) Western blot analysis of HK in representative samples showing intact HK (120 kDa), HK light chain (56 kDa), and light chain fragment (45 kDa). Lane labeled “HK” is loaded with purified HK for positive control. (D) Intact HK levels normalized to transferrin were significantly lower in AD (P < 0.0001) than ND plasma. When AD cases with a history of stroke (n = 5) were excluded from the analysis, intact HK levels in AD SE remained significantly higher than in ND plasma (P = 0.0002). Red points in AD and AD SE groups represent individuals who developed cognitive decline at least 1 y after blood draw. (E) Kallikrein-like activity was higher in AD plasma compared with ND (P = 0.0006). (F) Kallikrein-like activity was inversely correlated to intact HK levels (r = −0.81; P < 0.0001). (G) HK levels normalized to transferrin were higher in both individuals with CERAD score B (P = 0.003) and those with CERAD score C (P < 0.0001) than in individuals with CERAD score 0. Samples were analyzed three separate times, with similar results. Results are presented as vertical scatter plots with medians, with statistical significance determined using the Mann–Whitney test for two-group comparisons and the Kruskal-Wallis test with Dunn’s Multiple Comparison posttest for comparisons between multiple groups.