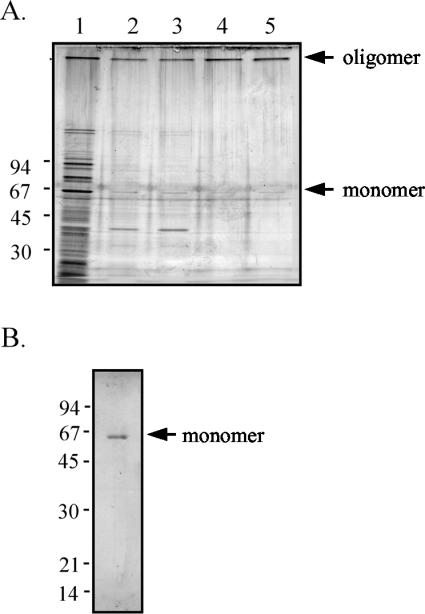
FIG. 1.
Purification of the YscC oligomer with the nonionic detergent Elugent. (A) Protein fractions of the different steps of the purification procedure were analyzed on a 3 to 15% polyacrylamide gradient gel and stained with silver. Samples were adjusted to contain equal amounts of YscC oligomer. Lane 1, protein fraction from whole cells (lane 1); lane 2, protein fraction from cell envelopes; lane 3, soluble protein fraction obtained after membrane extraction with Elugent; lane 4, protein fraction obtained after sucrose gradient centrifugation; lane 5, purified YscC oligomer after ion-exchange chromatography. (B) The purified YscC oligomer was dissociated with hot phenol, loaded on an 11% polyacrylamide gel, and visualized with Coomassie brilliant blue staining. The positions of the molecular mass markers (in kilodaltons) and of the YscC oligomer and monomer are indicated to the left and right of the gels, respectively.