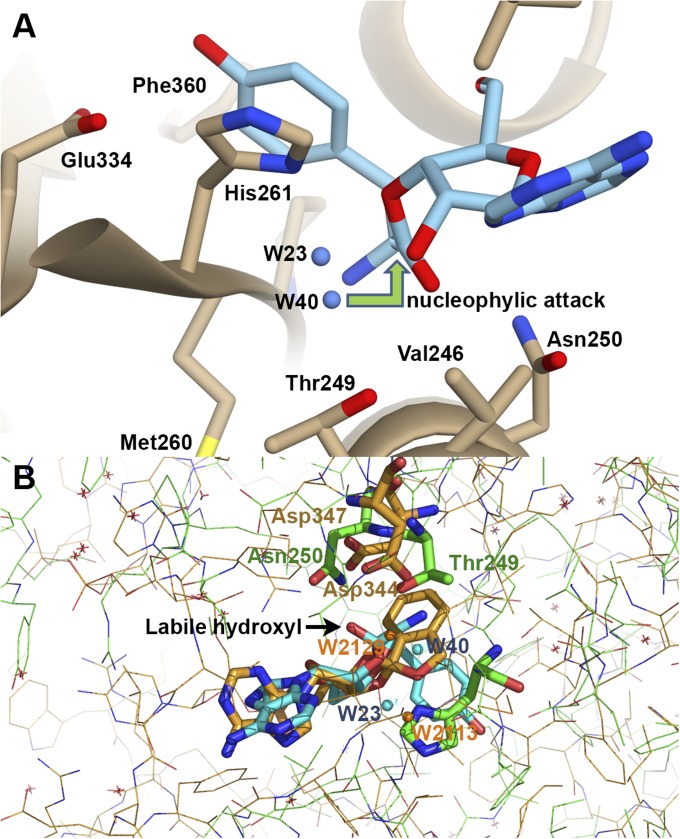
Fig. 4.
(A) The editing site of TtPheRS with modeled tyrosyl-3′-ribose ester (S1). The 3′-ester analog resembles the experimentally observed puromycin. Two water molecules, W23 and W40, are located on one side from the cleaved ester bond. (B) Superposition of the spiroborate/ribose structure of adduct (golden sticks) with two water molecules, W2113 and W2129, from editing site of LeuRS onto the orthoester moiety (blue sticks) with water molecules, W23 and W40, from TtPheRS editing site. Key residues Thrβ249 and Asnβ250 in TtPheRS are colored green, while the partner residues in LeuRS Asp344 and Asp347 are colored golden. Distances between W40-W2129 and W23-W2113 are 1.44 Å and 2.69 Å, respectively. Positions of the water molecule W2113 (LeuRS) and the ND1 atom of Hisβ261 (TtPheRS) are juxtaposed.