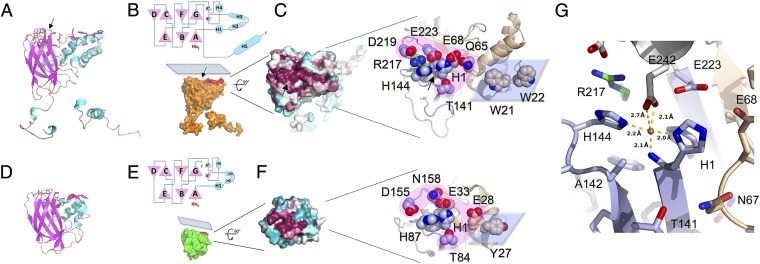
Fig. 2.
Fusolin has the hallmarks of a lytic chitin monooxygenase. Comparison of the fusolin and CBP21 proteins reveals similar 3D structures (A and D) and topologies (B, Upper and E, Upper) with a conserved flat platform on the molecular surface (B, Lower and E, Lower). Mapping of sequence variability onto the molecular surface revealed that the flat platform is highly conserved (C, Left and F, Left). The blue–white–red gradient represents low-to-high sequence conservation in 27 viral sequences (C) and 400 CBP21-like proteins (F). The two LPMO active sites are represented (C, Right and F, Right). Side chains of residues mentioned in the text are shown as spheres. The projection and Fn3 domains are colored in brown and light blue, respectively; Arg217 in green; and the polar rim and flat platform in pink and blue, respectively. (G) Representation of the metal-binding site in MMEV spindles with the same color scheme as C. A neighboring molecule capping the site is shown in white. Although this site is only occupied by a water molecule in purified MMEV spindles (Fig. S5), it can be populated by a Cu2+ ion after incubation in CuSO4, shown here as a brown sphere. The metal-binding site also is indicated by black arrows in A, B, and C.