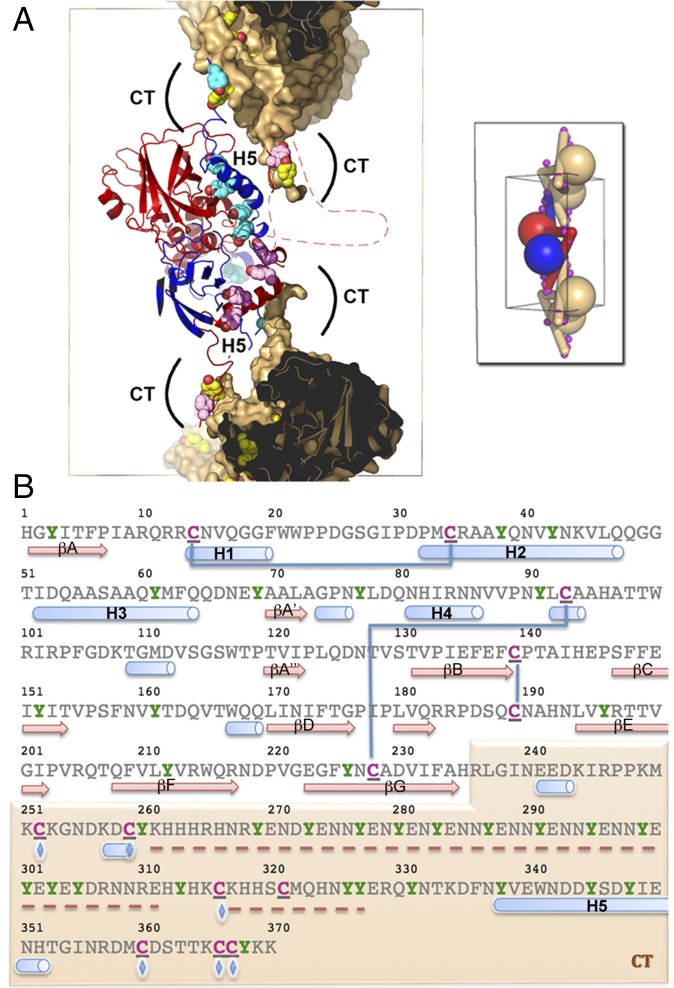
Fig. 4.
The CT extension controls the stability and dissociation of spindles through intermolecular disulfide bonds and multiple tyrosine residues, respectively. (A) Deprotonated tyrosines located at dimeric interfaces and crystal contacts may participate in the dissociation of spindles in alkaline conditions. The central dimer is shown as ribbons colored in blue and red. The molecular surface of the capping dimers is shown in brown but is omitted for CT tyrosine residues. The side chains of tyrosine residues in the CT region (including the H5 helix) are represented as spheres colored according to the rest of the molecule. The front part of the molecules has been clipped to reveal the interface. Regions clipped and absent from the model are indicated by a dashed line. (Inset) A schematic representation of connectivity. Magenta spheres indicate intermolecular disulfide bonds that crosslink spindles. (B) Sequence of the MMEV fusolin highlighting cysteine and tyrosine residues in magenta and green, respectively. These residues are particularly abundant in the CT arm shaded in brown. Blue lines represent intramolecular disulfide bonds, and blue diamonds indicate residues involved in intermolecular disulfide bonds (C252–C366; C259–C360; C316–C367). Secondary structure elements are shown under the sequence with missing regions indicated by a red dashed line.