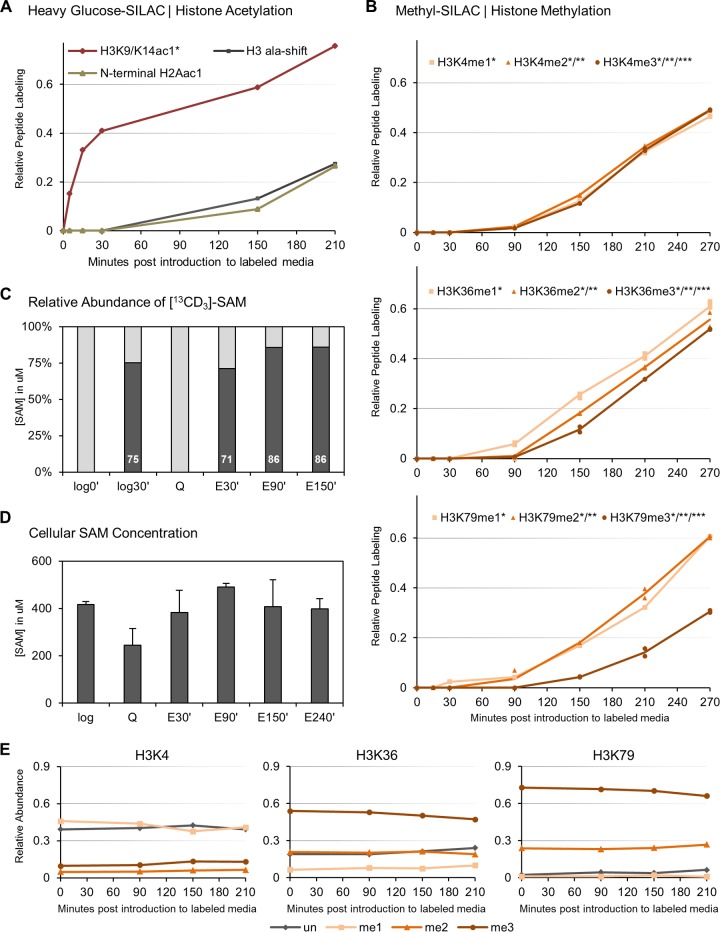
FIG 3.
Histone methylation, unlike histone acetylation, remains static during quiescence exit. (A) [13C6]glucose-SILAC shows an immediate nutrient-induced increase in labeling of histone H3 acetylation (H3K9/K14ac1*). N-terminal histone H2A acetylation is mediated cotranslationally and becomes labeled only late in exit, concurrently with alanine labeling of newly synthesized histone proteins. (B) Methyl-SILAC during quiescence exit shows that all H3 K4, K36, and K79 methylation states remain remarkably static during nutrient-induced cell cycle reentry, and new methylation occurs only late in exit concomitantly with histone protein synthesis. (C) Mass spectrometry confirms rapid labeling of cellular SAM pools by [13C]D3-methionine in proliferating cells (log0′ and log30′) and upon nutrient replenishment in quiescent cells when histone methylation remains static. (D) Levels of methyl donor SAM were assessed. Cellular SAM levels are slightly lowered in quiescence but rise during early quiescence exit to levels characteristic of cycling cells. (E) Mass spectrometry shows that the overall levels of the corresponding methylated H3 peptides remain constant.