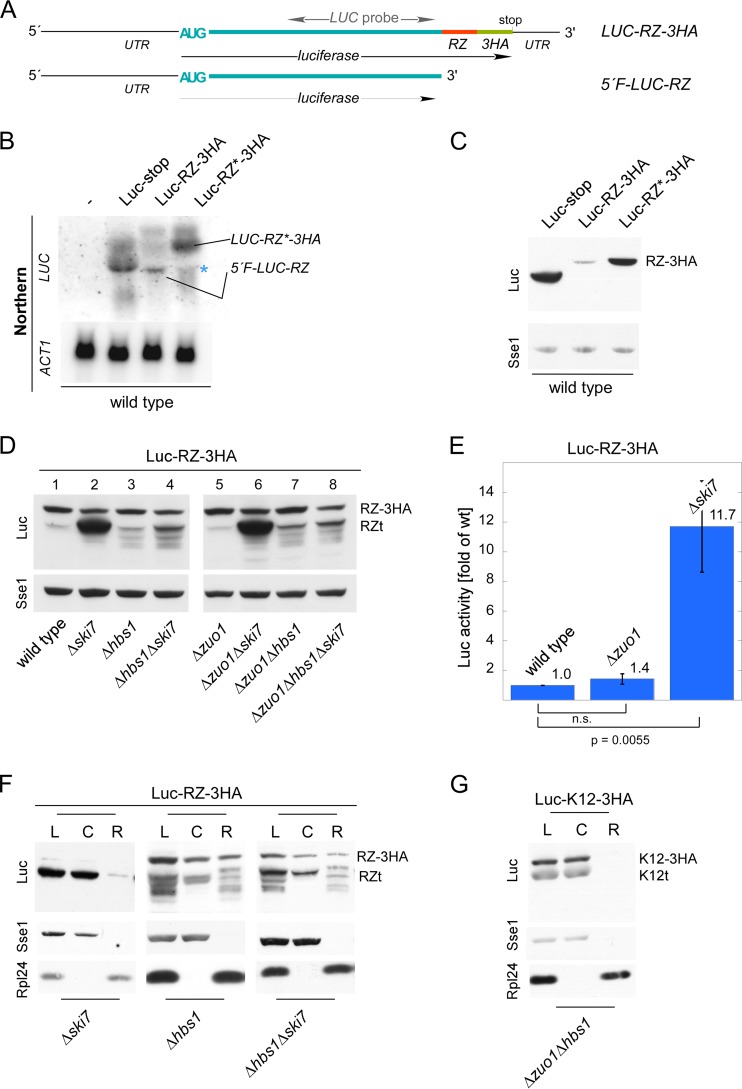
FIG 3.
Expression of stop codon-less proteins is independent of RAC/Ssb. (A) Luc-RZ-3HA reporter. Luciferase was fused in frame to the hammerhead ribozyme (RZ) sequence, followed by a sequence encoding three consecutive HA tags (LUC-RZ-3HA). RZ undergoes autocatalytic cleavage, generating a stop codon-less 5′ fragment (5′F-LUC-RZ). The position of the Northern probe employed in panel B is indicated (see Fig. S1 in the supplemental material). (B) The hammerhead ribozyme sequence induces mRNA cleavage. Total RNA isolated from the wild-type strain expressing Luc-stop, Luc-RZ-3HA, or Luc-RZ*-3HA was analyzed via Northern blotting using a probe recognizing luciferase (LUC). The wild type without a luciferase reporter served as a control for probe specificity. Actin (ACT1) served as a loading control. The positions of LUC-RZ-3HA/LUC-RZ*-3HA and 5′F-LUC-RZ are indicated. The asterisk indicates a background band. (C) The 5′F-LUC-RZ fragment is not efficiently translated. Total extracts of the wild type expressing luciferase reporters as indicated were analyzed via immunoblotting with antibodies recognizing luciferase (Luc), and as a loading control, Sse1. The product of LUC-RZ-3HA/LUC-RZ*-3HA translation (RZ-3HA) is indicated. (D) The Δhbs1 and Δski7 mutations, but not the Δzuo1 mutation, affect expression of 5′F-LUC-RZ. The indicated strains harboring the Luc-RZ-3HA reporter were analyzed as described above for panel C. The products of LUC-RZ-3HA translation (RZ-3HA) and of 5′F-LUC-RZ translation (RZt) are indicated. (E) Luc-RZt is catalytically active. Luciferase activity was determined in lysates of the indicated strains expressing Luc-RZ-3HA. Luciferase activity in the wild-type strain was set at 1. The averages of 4 independent experiments are shown; error bars represent the standard errors of the means. P values were calculated via Student's t test. n.s., not significant. (F) Ribosome release of Luc-RZt requires Hbs1. Lysates (L) derived from strains expressing Luc-RZ-3HA were separated into a cytosolic supernatant (C) and ribosomal pellet (R). Analysis was performed as described in the legend to Fig. 2A. Because of the high level of expression of Luc-RZt (see panel D also) in the Δski7 strain, only 25% of the aliquots was loaded. (G) Ribosome release of Luc-K12t does not require Hbs1. Lysate (L) derived from the Δzuo1Δhbs1 strain expressing Luc-K12-3HA was separated into a cytosolic supernatant (C) and a ribosomal pellet (R). Analysis was performed as described in the legend to Fig. 2A.