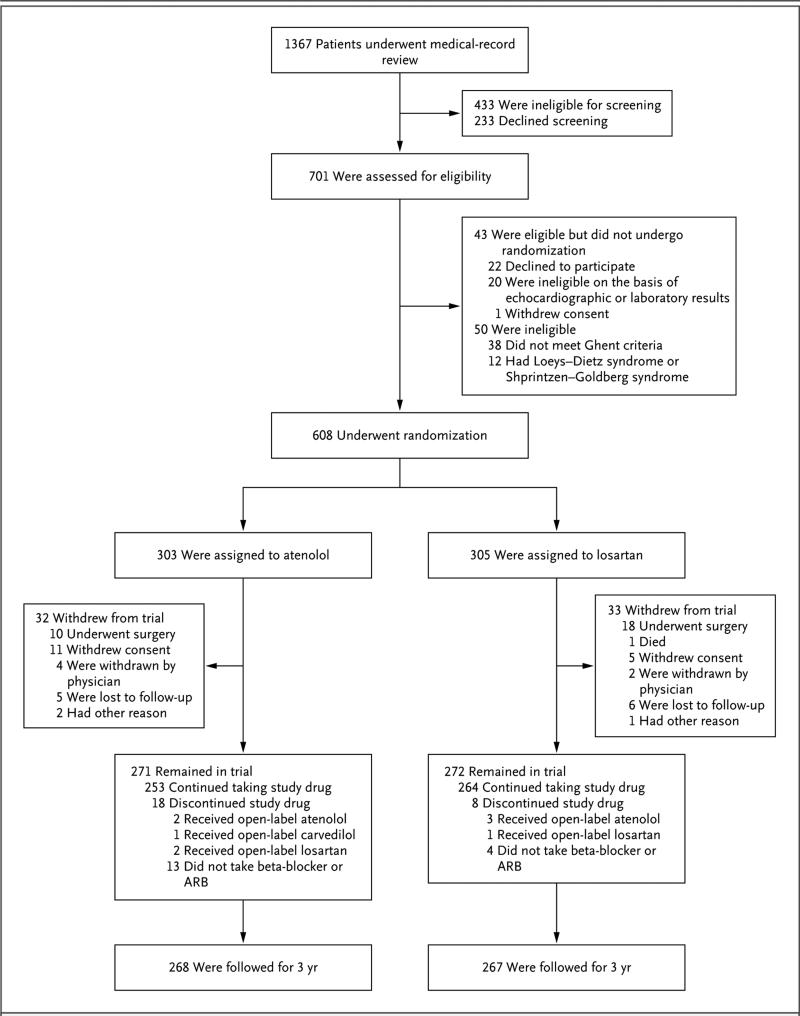
Figure 1. Screening, Randomization, and Follow-up.
The most common exclusion criterion during the medical-record review (71% of records reviewed) was an aortic-root-diameter z score of less than 3.0. Among the 543 participants who remained in the trial, data for the primary end point at 3 years were available for all but 11, who had unacceptable or missing echocardiograms (5 participants in the atenolol group and 6 in the losartan group). In addition, 3 participants withdrew late from the study (2 participants in the atenolol group because of aortic-root surgery and 1 in the losartan group because of unplanned pregnancy); the echocardiograms obtained at the time of withdrawal for these 3 participants served as the 3 year measurements, yielding a total of 535 participants with data for the primary end point at 3 years (268 participants in the atenolol group and 267 in the losartan group). ARB denotes angiotensin II–receptor blocker.