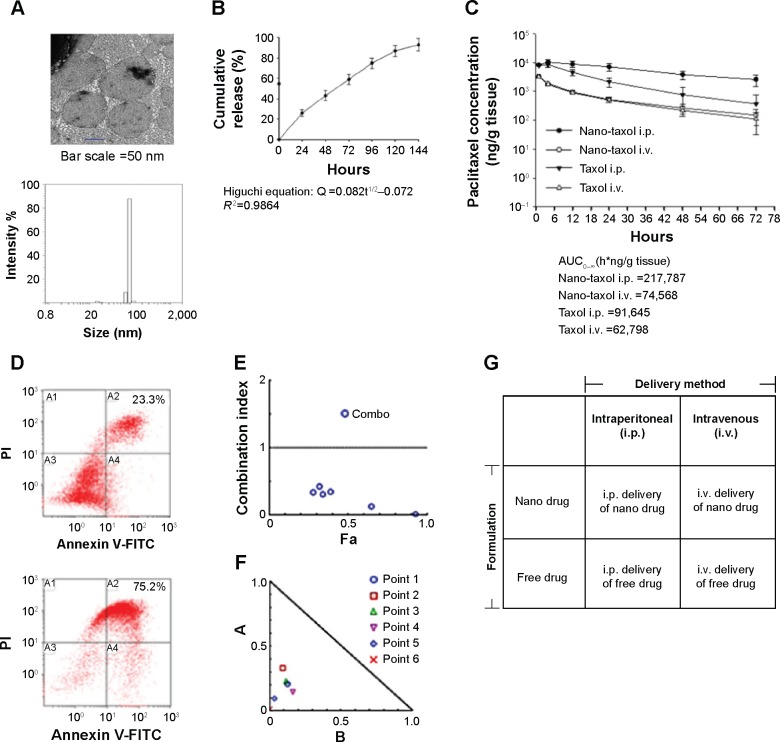
Figure 1.
Characteristics and synergism of sustained-release Nano-taxol.
Notes: (A) The unilamellar morphology of the liposomes was demonstrated by transmission electron microscopy (upper panel). The liposomes were estimated to have an average size of 100 μm by dynamic light scattering (lower panel). (B) Mathematical processing of the in vitro release data showed that the release of paclitaxel from liposomes obeyed Higuchi release kinetics (square root of time, R2=0.9864), indicating a diffusion-controlled model. (C) Intratumor drug concentration of Nano-taxol versus Taxol® by systemic or intraperitoneal delivery using an in vivo xenograft model. The intraperitoneal delivery of Nano-taxol achieved the highest AUC0–∞. (D) A flow cytometry assay of apoptosis using an in vivo xenograft model was used to compare the intraperitoneal delivery of Nano-taxol with the systemic delivery of this drug, which is the current standard therapy for ovarian cancer. Early and late apoptosis was significantly higher in the former group (75.2%) than in the latter group (23.3%). (E, F) Chou–Talalay plot showing the combined indices on the y-axis as a function of the effect levels (Fa) on the x-axis (E) and the normalized isobologram (F) using an in vivo xenograft model. The drug combination indices of six experimental points were all less than 1, indicating a synergistic effect between the nanomedicine and regional delivery. Values below the threshold line represent synergistic combinations. (G) A factorial analysis (two-way analysis of variance) was conducted to test the effect of the delivery method (intraperitoneal versus intravenous) and the drug formulation (nanomedicine versus free drug). The experiments were performed in triplicate.
Abbreviations: AUC, area under the concentration-time curve; i.p., intraperitoneal; i.v., intravenous; FITC, fluorescein isothiocyanate; PI, propidium iodide.