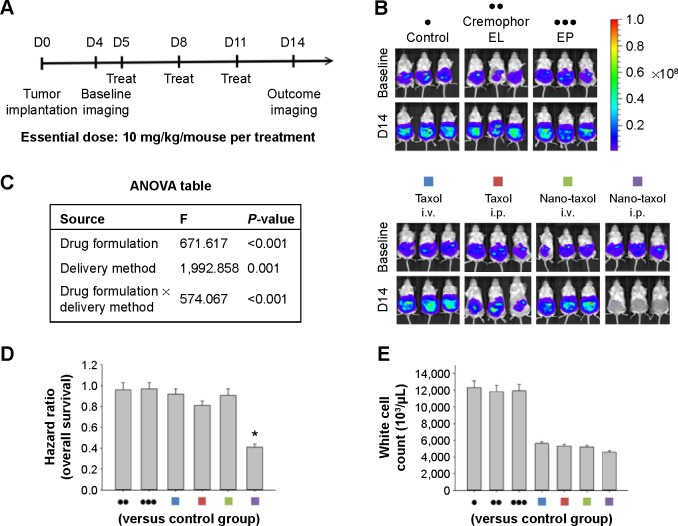
Figure 2.
Therapeutic effects of intraperitoneal delivery of Nano-taxol.
Notes: (A) Treatment schema. Both Taxol® and Nano-taxol were administered at an essential dose of 10 mg/kg/mouse per treatment. (B) Evaluation of therapeutic efficacy. The mice were implanted with ES-2 cells (2×105 per mouse) on day 1 and were treated on days 5, 8, and 11 with the indicated doses. Bioluminescence images were captured on day 4 (baseline) and day 14. Intraperitoneal delivery of Taxol shows some efficacy, but intraperitoneal delivery of Nano-taxol demonstrates the best tumor-killing efficacy. Cremophor EL is a vehicle for paclitaxel. (C) Factorial two-way ANOVA table showing effect of delivery method, drug formulation, and their interactions in terms of therapeutic efficacy. (D) Summary of the hazard ratios of overall survival in each group. Intraperitoneal delivery of Nano-taxol shows a hazard ratio of only 0.4 (*P<0.05). (E) White cell counts in each group. The mean white cell count obtained by intraperitoneal delivery of Nano-taxol was not significantly different from that obtained by systemic delivery of Taxol. The experiments were performed in triplicate.
Abbreviations: ANOVA, analysis of variance; EP, empty particles; i.p., intraperitoneal; i.v., intravenous.