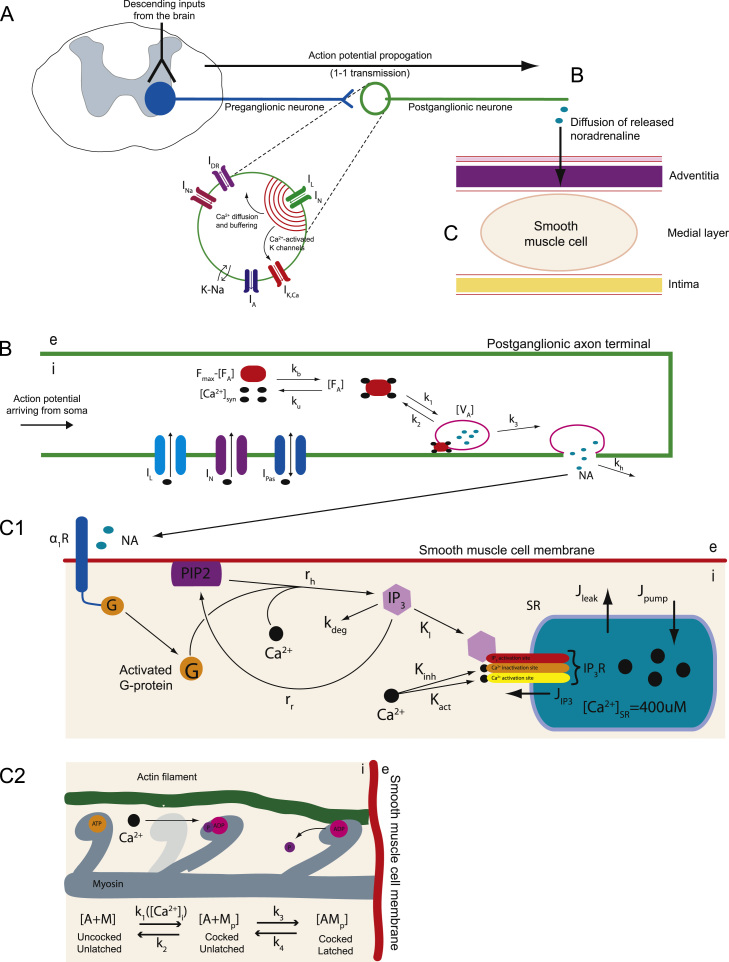
Fig. 1.
The mathematically modelled pathway from SPGN excitation to smooth muscle cell activation. The mathematically modelled pathway from SPGN excitation to smooth muscle cell activation. (A) The model of a SPGN from Briant et al. (2014). Action potentials propagate down the SPGN axon to the postganglionic terminal. (B) This activates IL and IN, triggering the influx of calcium into the postganglionic terminal, increasing . Four molecules of intracellular calcium bind to a fusion protein, activating it (FA). Once activated, the fusion protein can bind to, and consequently activate, a vesicle V. The activated vesicles, VA, are assumed to be pre-docked to the synaptic membrane; once activated, it immediately releases its NA contents into the cleft. (C1) Released noradrenaline activates α1Rs on the SMC membrane, activating a G-protein (G). This G-protein, drives the hydrolysis of PIP2. Hydrolysed PIP2 cleaves to form IP3, which activates an IP3R located on the membrane of the SR. Activation of this receptor causes an efflux of Ca2+ from the SR (JIP3), increasing . These receptors also have inactivation and activation sites for . Fluxes of Ca2+ across the SR membrane also exist due to leakage (Jleak) and calcium pumps (Jpump). (C2) The intra-SMC matrix contains actin (A) and myosin (M) filaments. At rest these filaments are in a detached state, . When the myosin heads are phosphorylated by calcium (MP), they able to attach to the actin filaments, yielding the state AMP—a cross-bridge. This cross-bridge can then conduct a ‘power stroke’, sliding the actin filament and producing a contractile force.