Abstract
Culex mosquitoes have emerged as important model organisms for mosquito biology, and are disease vectors for multiple mosquito-borne pathogens, including West Nile virus. We characterized epoxide hydrolase activities in the mosquito Culex quinquefasciatus, which suggested multiple forms of epoxide hydrolases were present. We found EH activities on epoxy eicosatrienoic acids (EETs). EETs and other eicosanoids are well-established lipid signaling molecules in vertebrates. We showed EETs can be synthesized in vitro from arachidonic acids by mosquito lysate, and EETs were also detected in vivo both in larvae and adult mosquitoes by LC-MS/MS. The EH activities on EETs can be induced by blood feeding, and the highest activity was observed in the midgut of female mosquitoes. The enzyme activities on EETs can be inhibited by urea-based inhibitors designed for mammalian soluble epoxide hydrolases (sEH). The sEH inhibitors have been shown to play diverse biological roles in mammalian systems, and they can be useful tools to study the function of EETs in mosquitoes. Besides juvenile hormone metabolism and detoxification, insect epoxide hydrolases may also play a role in regulating lipid signaling molecules, such as EETs and other epoxy fatty acids, synthesized in vivo or obtained from blood feeding by female mosquitoes.
1. Introduction
Epoxide hydrolases (EHs) are enzymes that convert a variety of epoxides into their corresponding diols (Morisseau and Hammock, 2005). In insects, epoxide hydrolases are mainly studied as detoxification enzymes (Dauterman, 1982; Mullin, 1988; Taniai et al., 2003), and enzymes that are involved in the metabolism of juvenile hormones (Anspaugh and Roe, 2005; Casas et al., 1991; Keiser et al., 2002; Khalil et al., 2006; Seino et al., 2010; Severson et al., 2002; Tsubota et al., 2010; Zhang et al., 2005). It is not known whether insect epoxide hydrolases play other important roles in insect physiology, and what other substrates can be involved.
In mammals, epoxides of fatty acids such as epoxyeicosatrienoic acids (EETs) are a group of eicosanoids that are lipid signaling molecules. EETs are derived from arachidonic acids, and are mainly hydrolyzed by the soluble epoxide hydrolase (Yu et al., 2000; Zeldin et al., 1993). Inhibition of soluble epoxide hydrolase revealed therapeutic effects in several mammalian models, indicating EETs are biologically functional (Morisseau and Hammock, 2013). In invertebrates including insects, eicosanoids are also known to play physiological roles such as ion transport, immunity, reproduction and host-vector interactions, although most studies had focused on prostaglandins (Stanley, 2006; Stanley and Kim, 2014; Stanley and Miller, 2006). It remains unknown whether insects produce EETs that are metabolized by epoxide hydrolases, and what the biological roles are.
Culex mosquitoes are widely distributed around the world, both in tropical and subtropical areas (Diaz-Badillo et al., 2011). They feed on a variety of hosts and are vectors of many important mosquito-borne diseases, such as West Nile virus (Bartholomay et al., 2010). Mosquitoes need arachidonic acids as the essential fatty acids, and replacement of arachidonic acids with prostaglandins cannot rescue the mosquitoes, indicating other metabolites of arachidonic acids may be important (Dadd, 1980; Dadd and Kleinjan, 1984). Mosquitoes may oxidize arachidonic acids to form EETs by monooxygenases, such as the cytochrome P450 in mammals (Capdevila et al., 1992; Zeldin, 2001), and female mosquitos will also ingest xenobiotic EETs during the process of blood feeding, because EETs and other epoxy fatty acids are regular components in the blood (Jiang et al., 2012; Jiang et al., 2005). Many blood-derived molecules have been found and studied. When ingested by mosquitoes, some are still relatively stable, and can affect mosquitoes’ capacity as disease vectors (Pakpour et al., 2013). As a result, EETs potentially may be among these molecules that have impacts on mosquito physiology and host-vector interactions.
Here we characterized the EH activities in the mosquito Culex quinquefasciatus, and demonstrated epoxy fatty acids are endogenous substrates. We showed that mosquitoes can produce epoxy fatty acids in vivo, and female mosquitoes will obtain xenobiotic epoxy fatty acids during blood feeding. The highest EH activities on EETs in adults were found in female midgut, which is the center of many physiological processes, including detoxification, digestion and molecular signaling. We also found urea-based sEH inhibitors can inhibit EH activities on EETs in mosquitoes. These inhibitors have been demonstrated to play diverse biological roles in mammalian systems (Shen and Hammock, 2011), which may be useful tools to evaluate epoxy fatty acid functions in mosquitoes.
2. Materials and Methods
2.1. Mosquito rearing
The mosquito C. quinquefasciatus were reared in an insectary incubator at a constant temperature of 28 ± 1°C and 80 ± 5% relative humidity. Eggs were hatched in plastic water cups, and larvae were fed twice a day with grounded fish food (TetraMin, Germany) and cat food (Purina, MO) until pupation. Emerged adults were transferred to mosquito cages (30 cm × 30 cm ×30 cm) and fed 10% sucrose ad libitum soaked in cotton balls daily. Three or four days after eclosion, mosquitoes were fed with defribrinated sheep blood (Quad Five, MT) at 37°C for 30 minutes. Parafilm® M (Sigma-Aldrich, MO) was used as the artificial membrane for blood feeding. After blood feeding, mosquitoes were provided with 10% sucrose daily, and water cups were provided for egg laying two days after blood feeding.
2.2. Enzyme preparation
4th instar larvae (8–9 days old after hatch) and adult mosquitoes (4–7 days female after eclosion) were homogenized by ceramic pestle and mortar in cold homogenization buffer (pH 8, 50 mM Tris-HCl buffer containing 1 mM phenylmethylsulfonyl fluoride and 1mM ethylenediaminetetraacetic acids). Because we were specifically interested in the EH activities in female mosquitoes, only female adults were selected. The whole mosquito extract was subjected to 100×g centrifugation for 5 minutes to remove debris. The supernatant was collected as the crude lysate. The mitochondria fraction was obtained by centrifuging the lysate at 18,000×g for 20 minutes, and the resulting pellets were resuspended in 50 mM, pH 8 Tris-HCl buffer. The resulting supernatant was centrifuged again at 100,000×g for 1 hour. The supernatant was collected as the cytosolic fraction, and the pellet was resuspended in Tris-HCl buffer as the microsomal fraction. The pellets in each step were washed once by homogenization buffer before any further processing. All differential centrifugations were processed at 4 °C. Protein concentration was measured by BCA assay (Pierce, Rockford, IL) with BSA (Pierce, Rockford, IL) as the standard throughout this study.
2.3. Measurement of epoxide hydrolase activity
Epoxide hydrolase activities on c-SO, t-SO, t-DPPO, c-DPPO, JH III, 14,15-EET and 9,10-EpOME were measured as previously described (Morisseau, 2007). To inhibit GST activity, diethyl maleate was added to the buffer at 1 mM final concentration. In the assays on JH III, 1 µl of 1 mM OTFP (3-octylthio-1, 1, 1-trifluoropropan-2-one) in ethanol was added into 100 µl enzyme solution (10 µM final concentration of OTFP) to inhibit JH esterase activity (Abdel-Aal and Hammock, 1985). Assays were done in triplicate, and all enzyme activities were corrected for non-enzymatic hydration. If inhibitors were added, different concentrations of inhibitors in DMSO were prepared, and 1 µl of inhibitor solution was added into 100 µl of lysate. 1 µl of DMSO was added in control assays although inhibition by DMSO was not observed at 1% (v:v) concentration.
2.4. Separation of epoxide hydrolase activities by ion exchange chromatography
A preliminary small-scale batch adsorption was used to define the loading and eluting conditions. DEAE Sepharose ion exchanger (1 ml) (GE Healthcare, UK) was added into a 10 ×75 mm borosilicate glass tube. The DEAE ion exchanger was equilibrated with pH 8, 20 mM, Tris-HCl containing 0.02% CHAPS by washing the gel five times with 10 ml of buffer. A known amount of enzyme solution solubilized by 0.02% CHAPS was added to the pre-equilibrated exchanger. The solution and gel was gently mixed, and the gel was allowed to settle. Proteins were eluted by step-wise elution by adding 1ml of pH 8, 20 mM Tris-HCl buffer containing increasing concentration of NaCl, ranging from 0.05M, 0.1M, 0.2M, 0.5M and 1.0M NaCl. Every fraction was collected, and assayed for epoxide hydrolase activities with t-DPPO, JH III and 14,15-EET as the substrates. To achieve better separation, a column and gradient elution was used. A batch of DEAE ion exchanger was equilibrated as previously described, and a known amount of enzyme solution was added into the exchanger. The whole solution was poured into the column. The outlet valve was opened to allow the gel to pack. The column was washed by 5 column volumes of pH 8, 20 mM Tris-HCl buffer containing 0.02% CHAPS to elute unbound materials. Bound proteins were eluted by 30 column volumes of NaCl gradient (200 mM to 1M). At last 5 column volumes of 1M NaCl was used to elute any remaining materials on the column. Every 10 ml elutant was collected for enzyme activity assays.
2.5. Synthesis of EETs by mosquito crude lysate
The mosquito 4th instar (8–9 days old after hatch) crude lysate (2 mg/mL) may be purged with CO for 2 minutes (one bubble per second) or added EH inhibitor AUDA to a final concentration of 1 µM. Then 1 µl of 10 mM arachidonic acid in ethanol (100 µM final concentration) was added into 95 µl mosquito crude lysate (2 mg/mL) in pH 8, 50 mM Tris-HCl buffer with or without premixed 5 µl of NADPH generating system (100 mM glucose-6-phosphate, 20 mM NADP+, 20 units glucose-6-phosphate dehydrogenase). Borosilicate culture tubes (ThermoFisher Scientific, MA) were used for the assay. The resulting solution was incubated at 30 °C in a shaking water bath, and the reaction was stopped by adding 400 µl methanol at 5, 10, 20 and 30 minutes respectively. CUDA (1 µl of 100 µM in methanol) was added into the solution as the internal standard (200 nM CUDA in a total volume of 500 µl solution). The tubes were vortexed for 5 seconds, and centrifuged at 2,500×g for 5 minutes. The supernatant was collected for LC-MS/MS analysis.
2.6. Mosquito dissection
Adult mosquitoes (4–7 days after eclosion) were anesthetized in a 4°C freezer, and transferred to a Petri dish on ice to keep immobilized. Mosquito legs were pulled off by hand. A drop of cold phosphate buffered saline was placed onto a glass slide mounted under the microscope, and mosquitoes were transferred to the slide. To get midguts and Malpighian tubules, a needle-tip probe was used to hold down the thorax, and a fine-tipped forcep was used to grasp the end of abdomen to gently pull off the abdomen in a slow motion. The midgut remained intact and attached to the thorax segment. The Malpighian tubules are long and slender tubules that are located between midgut and hindgut. To obtain salivary glands, a probe was used to hold down the thorax, and another probe was used to gently push down the anterior part of thorax, where the salivary gland is located. The dissected mosquito tissues were homogenized by a Pyrex® 1 ml glass tissue grinder (Sigma-Aldrich, MO) in pH 8, 50 mM Tris-HCl buffer.
2.7. Sampling, lipid extraction and LC-MS/MS
Mosquito 4th instar larvae (8–9 days old after hatch) or 4–7 days old adults (female only) were weighed and put in a 1.5 mL eppendorf tube. 10 µl of anti-oxidant solution (0.2 mg/ml of butylated hydroxytoluene and EDTA) and 10 µl of deuterated standards were added. 400 µl of ice-cold methanol with 0.1% of acetic acid and 0.1% of butylated hydroxytoluene were also added. Samples were stored in a −80°C freezer for 30 minutes. After freezing, samples were homogenized with a plastic pestle and stored in a -80°C freezer overnight. The next day the samples were centrifuged at 10,000×g for 10 minutes. The supernatant was collected, and the remaining pellets were washed with 100 µl of ice-cold methanol with 0.1% of acetic acid and 0.1% of butylated hydroxytoluene. The tubes were centrifuged again. The supernatant was combined and diluted with 1.5 ml of water. The resulting solution was loaded onto 60 mg Oasis HLB cartridges (Waters, MA).
The solid phase extraction (SPE) and LC-MS/MS analysis was followed as previously described (Yang et al., 2009). The eluted samples from SPE were evaporated by vacuum (SpeedVac) and reconstituted with 50 µl of 200 nM CUDA in methanol, which was used as the internal standard. Agilent 1200 SL liquid chromatography series (Agilent Corporation, CA) was used for separation with an Agilent Eclipse Plus C-18 reversed-phase column (2.1 × 150 mm, 1.8 µM particle size). Water with 0.1% glacial acetic acid was used as mobile phase A. Acetonitrile : methanol (84:16) with 0.1% glacial acetic acids was used as mobile phase B. The detection was carried out by monitoring the selected-reaction transitions using a 4000 QTrap tandem mass spectrometer (Applied Biosystems Instrument Corporation, CA) equipped with an electrospray source (Turbo V®). More detailed information of oxylipin analysis can be found somewhere else (Yang et al., 2009).
3. Results and Discussion
3.1. Biochemical characterization of EH activities
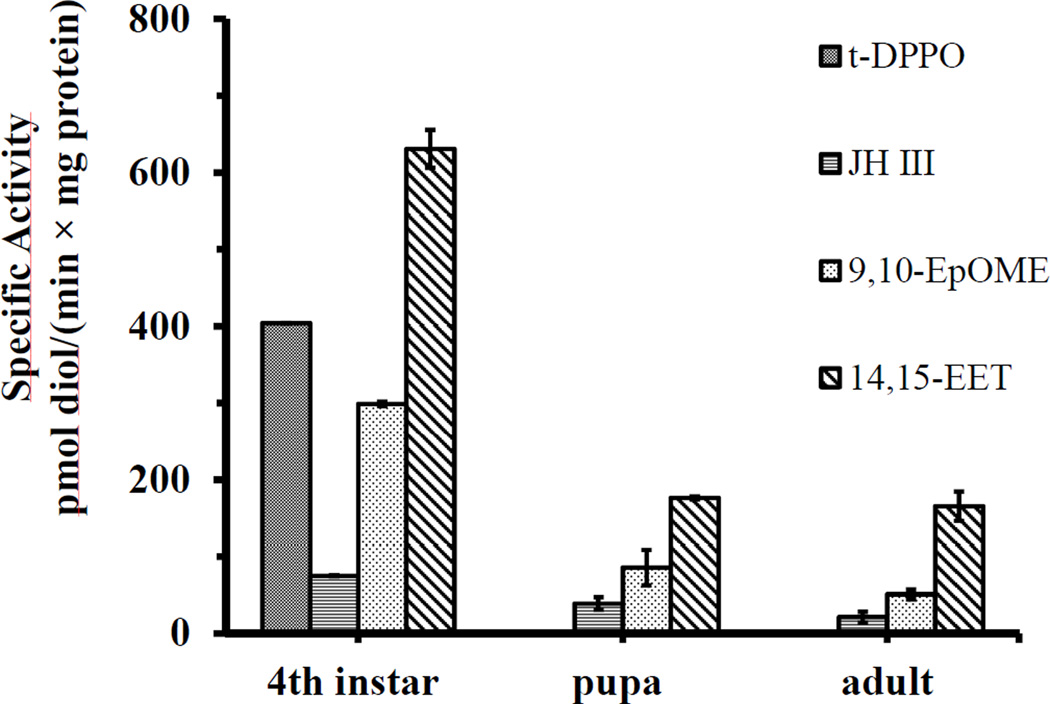
Among the substrates evaluated in the study, c-SO, t-SO, t-DPPO and c-DPPO are tritium-labeled compounds, which were surrogate substrates for EH activities. JH III is an endogenous substrate for EHs in insects. 14,15-EET and 9,10-EpOME are epoxides of arachidonic acid and linoleic acid respectively. The structure of the substrates is shown in Table 1. No EH activity on c-SO, t-SO and c-DPPO was found in 4th instar larvae (8–9 days old) (Table 1), while activities on JH III, 14,15-EET and 9,10-EpOME were detected. EH activity on t-DPPO was only detected in 4th instar larvae (8–9 days old after hatch), not in pupa (10–12 days old after hatch) and female adults (4–7 days old after eclosion) (Fig. 1). Activities on JH III, 14,15-EET and 9,10-EpOME were detected in larvae, pupa and adults (Fig. 1). The highest specific activity was found in the 4th instar larvae (Fig. 1), although the physiological significance of the difference is not known. In lysate of the 4th instar larvae, different levels of EH activities on four substrates were found in mitochondrial, cytosolic and microsomal fractions (Fig. 2), indicating distribution of EHs in multiple subcellular fractions. Solubilized EH activities on t-DPPO, JH III and 14,15-EET can all bind to the DEAE-Sepharose (GE Healthcare, UK) equilibrated by pH 8, 20 mM Tris-HCl buffer, and the activities were all eluted by 0.5M and 1.0M NaCl gradient by step elution (Fig. S1). When a NaCl gradient was used for elution, EH activities with different chromatographic characteristics were detected (Fig .3). The balance sheet for the purification is shown in Table S1. The activities on t-DPPO, JH III and 14,15-EET overlapped, but the peak for each substrate did not. Activities on four substrates in different developmental stages, subcellular locations and separation by ion exchange chromatography suggest the presence of multiple forms of epoxide hydrolases. They may have overlapping and complementary substrate selectivity, while sharing the same subcellular location.
Table 1.
Substrate selectivity of epoxide hydrolase activities from Culex quinquefasciatus.
| Substrates | Structure of substrates | Specific Activity (pmol diols formed/(min × mg protein)) |
|---|---|---|
| t-SO |  |
<30 |
| c-SO |  |
<30 |
| t-DPPO |  |
404±23 (<30) |
| c-DPPO |  |
<30 |
| 14,15-EET |  |
631±25 (<0.4) |
| 9,10-EpOME |  |
299±3 (<0.4) |
| JH III |  |
75±1 (<2) |
Fig. 1.
Epoxide hydrolase activities in different developmental stages of Culex quinquefasciatus. Enzyme activities were measured with triplicate assays. Values are mean activity ± SD based on three independent preparations. 4th instar (8–9 days old after hatch), pupa (10–12 days old after hatch) and female adults (4–7 days old after eclosion) were collected and homogenized for EH activity assays.
Fig. 2.
Subcellular distribution of EH activities in 4th instar larvae (8–9 days old after hatch) on four substrates. Values are mean activity ± SD based on three independent preparations. The mitochondria fraction was obtained by centrifuging the lysate at 18,000×g for 20 minutes, and the resulting pellets were resuspended in 50 mM, pH 8 Tris-HCl buffer. The resulting supernatant was centrifuged again at 100,000×g for 1 hour. The supernatant was collected as the cytosolic fraction, and the pellet was resuspended in Tris-HCl buffer as the microsomal fraction.
Fig. 3.
Epoxide hydrolase activities with different chromatographic characteristics. Each datum point is the mean of three independent chromatographic runs. The standard deviations are within 10% of the mean values and are not shown here. A column of 10 ml DEAE ion exchanger was used and 1 column volume is 10 ml of eluting buffer. The NaCl gradient was made by mixing 200 mM and 1 M NaCl in Tris-HCl buffer in a gradient maker. Gradient elution began at 5th column volume and ended at 35th column volume. EH activities in the first five column volumes (unbound enzyme fractions) were not detected. Protein concentration was measured by A280 with BSA as the standard. The binding and eluting conditions are shown in Fig. S1. The balance sheet for the purification is shown in Table S1.
Recently we have expressed and characterized an epoxide hydrolase from Anopheles gambiae in insect cells (AgEH) that has a high activity on epoxy fatty acids (Xu et al., 2014). When the protein sequence of AgEH was used as a query for blast in the genome of C. quinquefasciatus, we found five protein coding sequences with epoxide hydrolase signature elements (Arand et al., 1996; Hopmann and Himo, 2006; Morisseau and Hammock, 2005). We included the five sequences in a phylogeny analysis with reported mammalian and insect epoxide hydrolases. In the phylogeny analysis (Fig. 4), the five sequences were evolutionarily more close to mammalian EH 3, EH 4 and sEHs, a family of EHs that are involved in a variety of mammalian physiology by regulating the metabolism of epoxy fatty acids (Decker et al., 2012; Yu et al., 2000). They are remotely homologous to mammalian and insect microsomal EHs, including reported insect JHEHs (Seino et al., 2010; Severson et al., 2002; Taniai et al., 2003; Touhara et al., 1994; Zhang et al., 2005). Three putative JHEH sequences from C. quinquefasciatus were also clustered with insect JHEHs, but had a different evolutionary history with the five sequences mentioned previously. Interestingly, putative epoxide hydrolase sequences homologous to the mosquito AgEH and EHs from C.quinquefasciatus were also found in the genomes of Bombyx mori (BmEH), Apis mellifera (AmEH) and Tribolium castaneum (TcEH) (Fig. 4), suggesting mammalian EH 3, EH 4 and sEH homologs may be common in insects. Besides juvenile hormone epoxide hydrolases, there are other epoxide hydrolases in insects that may regulate insect physiology by metabolizing other important substrates (such as epoxy fatty acids) other than juvenile hormones. It is widely accepted that insects can synthesize fatty acids or obtain them from food resources (Dadd, 1981). Insects not only have the capacity to structurally alter the fatty acids, but also these fatty acid derived molecules may have different biological functions from their parent molecules (Stanley-Samuelson et al., 1988). These functions include components of insect exoskeleton, synthesis of pheromones (Stanley-Samuelson et al., 1988), reproduction and immunity (Stanley and Kim, 2014). Epoxides of fatty acids, as lipid signaling molecules, have been demonstrated to play a variety of roles in mammalian physiology and medicinal biology (Morisseau and Hammock, 2013), which are regulated by epoxide hydrolases (Morisseau, 2013). It is fundamental to express and characterize the putative epoxide hydrolases in B.mori, A. mellifera and T.castaneum, and determine whether insects and mammals share another conserved signaling pathway regulated by epoxide hydrolases.
Fig. 4.
Phylogeny analysis of EH sequences in Culex quinquefasciatus and other reported EHs. The full names of abbreviations and corresponding references are: AgEH, epoxide hydrolase from A. gambiae (Xu et al., 2014). HsEH, sEH from H. sapiens (Beetham et al., 1993); MsEH, sEH from M. musculus (Grant et al., 1993); RsEH, sEH from R. norvegicus (Knehr et al., 1993); EH 3, epoxide hydrolase 3 from H,sapiens (Decker et al., 2012); EH 4, epoxide hydrolase 4 from H.sapiens (Decker et al., 2012); CeEH1, sEH1 from C. elegans (Harris et al., 2008); HmEH, mEH from H. sapiens (Skoda et al., 1988); RmEH, mEH from R. norvegicus (Falany et al., 1987); DmEH, mEH from D. melanogaster (Taniai et al., 2003) ; BmJHEH-r1, JHEH-r1 from Bombyx mori (Seino et al., 2010); BmEH, putative epoxide hydrolase 4-like from Bombyx mori; AmEH, putative epxoide hydrolase 4-like from A.mellifera; TcEH, putative epoxide hydrolase 4 from T. castaneum. The accession number of amino acid sequences is shown in the parenthesis. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches.
The phylogeny analysis is consistent with our biochemical characterization that multiple forms of epoxide hydrolases exist in C. quinquefasciatus. The repertoire of protein-coding genes in C. quinquefasciatus was reported to be 22% and 52% larger than that of Aedes aegypti and Anopheles gambiae, respectively (Arensburger et al., 2010). One of the gene-family expansions is the expansion of genes associated with xenobiotic detoxification (Arensburger et al., 2010). Epoxide hydrolases historically were studied as detoxification enzymes, and enzymes that hydrolyze juvenile hormones in insects (Morisseau and Hammock, 2008). Multiple EHs expressed in C. quinquefasciatus can be a reflection of Culex-specific characteristics (Reddy et al., 2012), and EHs may play a role in metabolizing epoxy fatty acids, as their mammalian counterparts do.
3.2. EET and EpOME are endogenous substrates for mosquito epoxide hydrolases
We were particularly interested in the activities on epoxy fatty acids such as EpOMEs and EETs. EETs are epoxides of arachidonic acid, and have not been reported so far in insects to our knowledge. In mammals, EETs are lipid signaling molecules mainly hydrolyzed by soluble epoxide hydrolases (sEHs). The inhibition of sEH was shown to have therapeutic effects in several disease models, indicating EETs are anti-inflammatory, vasodilatory, angiogenic and analgesic (Morisseau and Hammock, 2013).
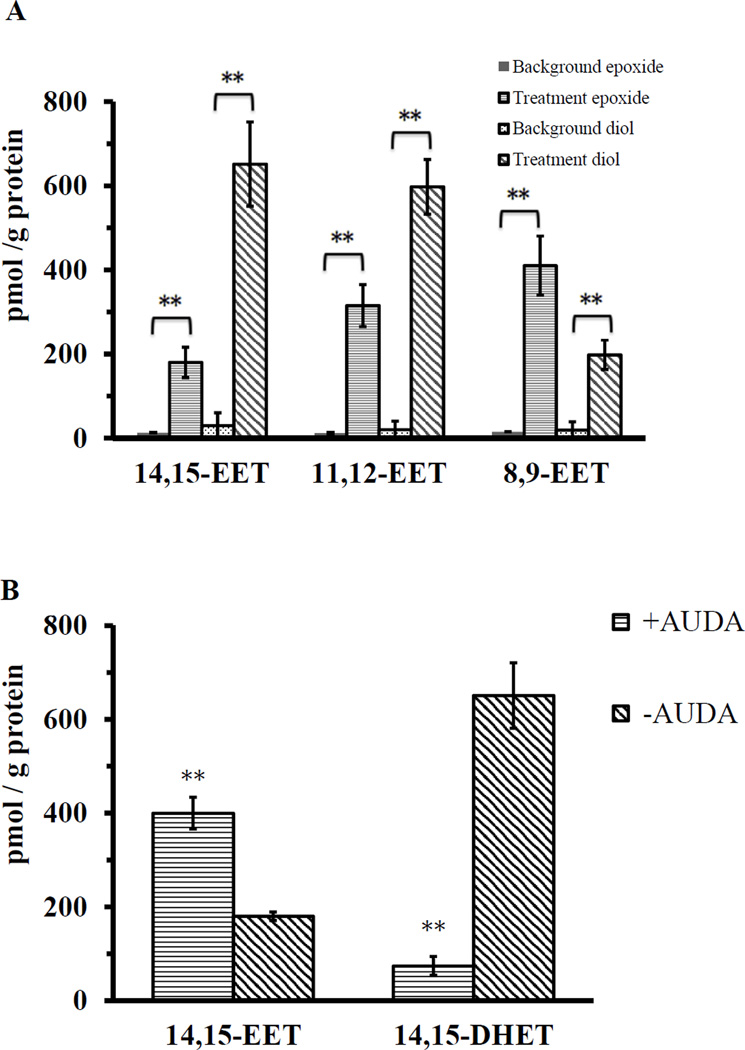
When arachidonic acids were added into the 4th instar (8–9 days old after hatch) crude lysate containing the premixed NADPH generating system, 8,9-EET, 11,12-EET, 14,15-EET and their corresponding diols were all detected by LC-MS/MS (Fig. 5A). The regioisomer 5,6-EET is not chemically stable (Fulton et al., 1998), and it is not reported here. The diols were also detected probably because of the epoxide hydrolase activities in the lysate. When 1 µM EH inhibitor AUDA was added into the reaction, 14,15-DHET (diols of 14,15-EET) detected decreased nine fold, and the amount of 14,15-EET detected was increased two fold (Fig. 5B). Using the regioisomer 14,15-EET as a representative of EETs, the largest amount of 14,15-EET was detected when NADPH generating system was added into the lysate (Fig. 5C). Interestingly, when the lysate was purged with CO or the NADPH generating system was not added, a lower amount of EET and diols was detected, but this amount was still significantly higher than the control groups (Fig. 5C). These data indicate cytochrome P450s are involved in the synthesis of EETs in vitro, but they are probably not the only source of EET production. During the 30-minute time course, the conversion rate from arachidonic acid to 14,15-EET and diols was 0.01%. Reasons for the low conversion may be arachidonic acid is the substrate for a variety of reactions, including β-oxidation, chain elongation and production of eicosanoids, leading to a small portion of arachidonic acid had been converted to EETs. Another possibility is that the EETs and their diols had been converted to other metabolites for which we have no standards for analysis.
Fig. 5.
Synthesis of EETs by mosquito 4th instar (8–9 days old after hatch) lysates in vitro. Student’s t test was used to evaluate statistical significance (** indicates p<0.01). Error bars represent the standard deviations of the means. A: Different regioisomers of EETs and corresponding diols detected by LC-MS/MS in 30 minutes of incubation. 5,6-EET was not included because its chemical instability. EETs and diols detected in the arachidonic acid solution and mosquito 4th instar (8–9 days old larvae) lysate only were regarded as background. In the treatment groups, arachidonic acid and NADPH generating system was added into mosquito 4th instar (8–9 days old larvae) lysate. B. Effects of a sEH inhibitor (AUDA) on the in vitro synthesis of 14,15-EET at 30 minutes of incubation. C: Synthesis of 14,15-EET with or without NADPH or CO in 30 minutes of incubation. The data of two control treatments ‘Buffer+ARA’ and ‘ Lysate only’ overlap and therefore only the data of ‘Lysate only’ is shown.
When mosquito 4th instar larvae (8–9 days after hatch) and adult samples (female only, 4–7 days after eclosion) were extracted for oxylipin analysis, all epoxides of linoleic acid, arachidonic acid and their corresponding diols were detected (Table 2). The concentration of EpOMEs was 45 fold and 25 fold higher than EETs in larvae and adult respectively. The concentration of DiHOMEs was 42 fold and 315 fold higher than DHETs in larvae and female adult.
Table 2.
Detection of epoxy fatty acids in vivo by LC-MS/MS. Mosquito 4th instar larvae (8–9 days old larva after hatch) or adults (female, 4–7 days old after eclosion) were weighted before extraction of lipid by solid phase extraction. Concentrations of C-18 and C-20 epoxy fatty acids and corresponding diols are presented as mean ± SD based on three independent measurements.
| Parent Fatty Acids |
Oxylipin | pmol/g larva | pmol/g adult | |
|---|---|---|---|---|
| Linoleic acid | Epoxy fatty acids | 9,10-EpOME | 9±2 | 11±1 |
| 12,13-EpOME | 6±2 | 10±1 | ||
| Dihydroxy fatty acids | 9,10-DiHOME | 12±4 | 150±30 | |
| 12,13-DiHOME | 16±3 | 90±20 | ||
| Arachidonic acid | Epoxy fatty acids | 8,9-EET | 0.20±0.04 | 0.25±0.08 |
| 11,12-EET | 0.07±0.02 | 0.35±0.09 | ||
| 14,15-EET | 0.08±0.02 | 0.30±0.05 | ||
| Dihydroxy fatty acids | 8,9-DHET | 0.18±0.05 | 0.10±0.04 | |
| 11,12-DHET | 0.15±0.04 | 0.12±0.05 | ||
| 14,15-DHET | 0.30±0.10 | 0.5±0.1 |
By in vitro EET synthesis and oxylipin analysis (Fig. 5 and Table 2), we demonstrated EETs, as well as EpOMEs, are the endogenous substrates for mosquito epoxide hydrolases. EETs and other eicosanoids have been extensively studied in terms of human health and drug development (Morisseau and Hammock, 2013; Tapiero et al., 2002). EETs have not been identified in insects, and their biology has not been reported to our knowledge. Although present in low concentrations, EETs and the corresponding diols clearly are endogenous molecules in mosquitoes. EETs and other epoxy fatty acids may also play physiological roles in mosquitoes as they do in mammals, which are regulated by epoxide hydrolases.
3.3. EH activities on 14,15-EET are highest in female midgut
To investigate the distribution of EH activities on EETs in mosquitoes, we dissected and homogenized a representative set of organs from 50 male or female (4–7 days old) adult mosquitoes. EH activities were measured with 14,15-EET as the substrate. There are sex differences in epoxide hydrolase activities on 14,15-EET in male and female mosquitoes (Fig. 6). The highest specific activities were found in the midgut of female mosquitoes. Intermediate enzyme activities were found in Malpighian tubules. The activities in the salivary gland, head and whole fly were relatively low in both sexes.
Fig. 6.
Epoxide hydrolase activities on 14,15-EET in different tissues of adult mosquitoes. Mosquitoes aged 4–7 days after eclosion were dissected under a microscope. Student’s t test was used to evaluate statistical significance (*p<0.01, **p<0.001) between tissues of males and females. Error bars represent the standard deviations of the means based on three independent measurements.
The sexual difference and tissue distribution suggest that the epoxide hydrolase activities on epoxy fatty acids may serve varying physiological roles in different tissues and sexes. Since only female mosquitoes need blood meals, and the midgut is the center for digestion, detoxification and pathogenic infection, it is tempting to hypothesize that the EH activities in the midgut on epoxy fatty acids are involved in midgut function, and may be important in terms of disease transmission.
3.4. EH activities on 14,15-EET can be induced by blood feeding
It was reported that blood feeding could modulate the expression of a variety genes (Dana et al., 2005; Sanders et al., 2003) in mosquitoes. To test whether the EH activities on epoxy fatty acids can be affected by blood feeding, we blood-fed the mosquitoes and sampled 10 mosquitoes every 3 hours for the measurement of EH activities. The epoxy fatty acid composition and EH activities of the sheep blood on epoxy fatty acids are shown in supplementary information (Table S2 and Fig. S2). Because 10 mosquitoes were homogenized in 1 mL pH 8, 50 mM Tris-HCl buffer, and one mosquito usually takes 1–2 µl of blood, the fatty acid components and EH activity from the blood (Fig. S2) would not interfere the EH activities measured in our experiments.
Because blood in the gut will interfere with protein concentration determination, the activity was measured as pmol diol formed per mosquito per minute. Non-ingested female mosquitoes were also sampled at every time point as controls. When not blood fed, the EH activities on 14,15-EET remained consistent (Fig. 7) during the course of sampling. After blood feeding, EH activities on 14,15-EET were induced 2 fold at 12 hours post blood feeding, and reduced to the same level as in the non-ingested females at 18 hours post blood feeding.
Fig. 7.
Induction of epoxide hydrolase activities on 14,15-EET after blood feeding. Each datum point represents means ± SD unless the SD is smaller than the datum point. Ten blood-ingested females were sampled every three hours after feeding, Non-ingested females were also sampled as controls. At 6, 9,12,15 hours post blood feeding, the differences were statistically significant (p<0.01 by Student’s t test) from the controls.
3.5. EH activities on epoxy fatty acids are inhibited by urea-derived compounds designed as mammalian sEH inhibitors
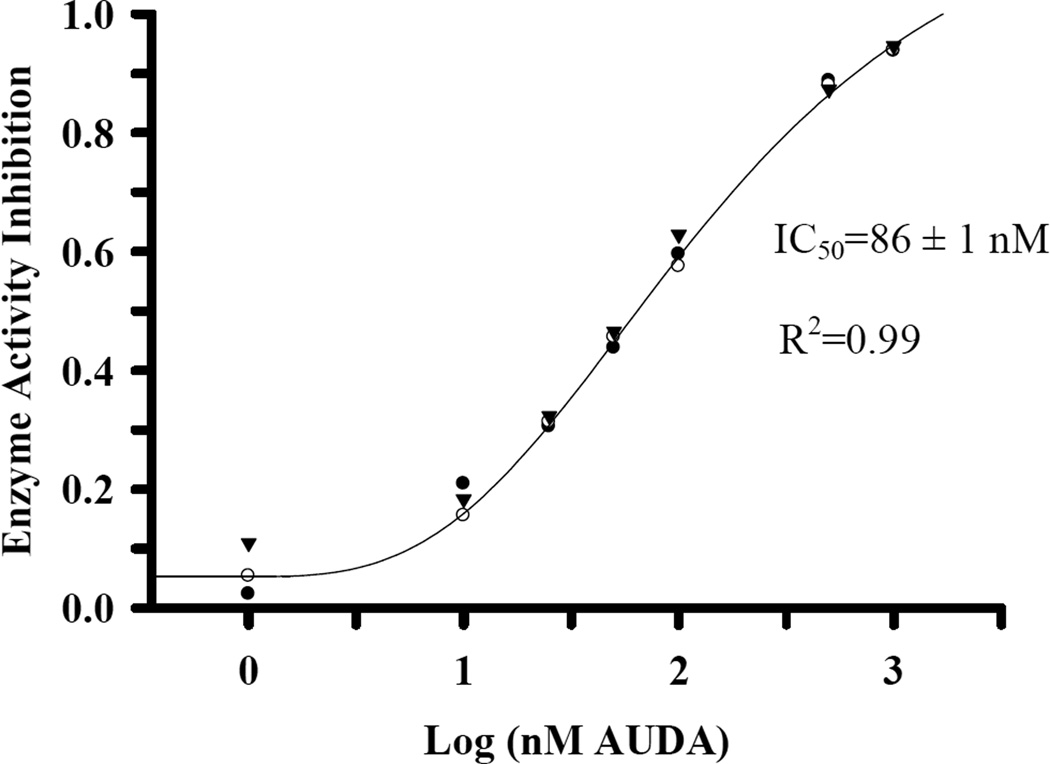
In mammalian systems, the soluble epoxide hydrolase dominates the metabolism of epoxy fatty acids. Although EETs are relatively stable, establishing their roles in vivo as signaling molecules was delayed due to their rapid hydrolysis by the sEH. Because we are interested in the biology of EHs and epoxy fatty acids, we tested a representative of EH inhibitors at relevant concentrations with 14,15-EET as the substrate. The structure of the inhibitors is shown in Table 3. At 1 µM concentration (Table 3), a potent mEH inhibitor (elaidamide) and most sEH inhibitors showed inhibition no greater than 30%, expressed by assigning the activities in control sample (only DMSO was added at 1% final concentration) as 0% inhibition. The inhibitor t-AUCB inhibited 65% activity, while AUDA showed a 97% inhibition. Both compounds were designed as EET mimics to inhibit the mammalian sEH. AUDA is the most close mimics of the EETs and has structural similarity on only one side of the urea central pharmacophore (Shen and Hammock, 2011). We selected AUDA and determined its IC50, which was around 86 nM (Fig. 8). We thus propose AUDA as an inhibitor for the epoxide hydrolases from C. quinquefasciatus to facilitate the investigation of the biological roles of epoxy fatty acids. It can also serve as a lead molecule for the synthesis of more powerful inhibitors of the EHs acting on epoxy fatty acids in insects.
Table 3.
Inhibition of epoxide hydrolase activities on 14,15-EET by EH inhibitors. Enzymes were extracted from 4th instar larvae (8–9 days old).
 
|
Fig. 8.
IC50 of AUDA on 14,15-EET. 4th instar (8–9 days old after hatch) lysate was incubated with different concentrations of AUDA, the most potent inhibitor identified in Table 3 for five minutes on ice. The symbols represent the mean of enzyme activity inhibition in three independent measurements. The IC50 is presented as mean ± SD based on three independent measurements. 1 l of 5 mM 14,15 EET in DMSO was added (50 M final concentration) for measuring enzyme activity. The 4 parameter logistic model describes the sigmoid-shaped response was used to calculate IC50 by the software SigmaPlot.
Supplementary Material
Multiple EH activities were characterized in the mosquito Culex quinquefasciatus.
Epoxy fatty acids are endogenous and xenobiotic substrates for EHs from mosquitoes.
AUDA is a useful inhibitor to investigate the biological roles of epoxy fatty acids.
Acknowledgement
This study was funded by NIEHS (R01 ES002710 and P42 ES004699), the West Coast Metabolomics Center at UC Davis (NIH/NIDDK U24 DK097154), the UC Davis Jastro-Shields Graduate Research Award and the China Scholarship Council. We thank Dr. Ahmet Inceoglu and Dr. Shizuo Kamita for detailed discussions and suggestions. We also thank Dr. Kin Sing Stephen Lee for the synthesis of 14,15-EET substrates.
Abbreviations
- EH
epoxide hydrolase
- JH
juvenile hormone
- sEH
soluble epoxide hydrolase
- JHEH
juvenile hormone epoxide hydrolase
- AgEH
epoxide hydrolase from A. gambiae
- HsEH
sEH from H. sapiens
- MsEH
sEH from M. musculus
- RsEH
sEH from R. norvegicus
- EH 3
epoxide hydrolase 3 from H. sapiens
- EH 4
epoxide hydrolase 4 from H. sapiens
- CeEH1
sEH1 from C. elegans
- HmEH
mEH from H. sapiens
- RmEH
mEH from R. norvegicus
- DmEH
mEH from D. melanogaster
- BmJHEH-r1
JHEH-r1 from Bombyx mori
- BmEH
epoxide hydrolase 4-like from Bombyx mori
- AmEH
epxoide hydrolase 4-like from A.mellifera
- TcEH
epoxide hydrolase 4 from T. castaneum
- c-SO
cis-stilbene oxide
- t-SO
trans-stilbene oxide
- t-DPPO
trans-diphenylpropene oxide
- EET
epoxyeicosatrienoic acid
- EpOME
epoxy octadecenoic acid
- AUDA
12-(3-adamantan-1-yl-ureido) dodecanoic acid
- t-TUCB
trans-4-{4-[3-(trifluoromethoxy-phenyl)-ureido]-cyclohexyloxy}-benzoic acid
- c-TUCB
cis-4-{4-[3-(trifluoromethoxyphenyl)-ureido]-cyclohexyloxy}-benzoic acid
- TPPU
1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea
- t-AUCB
trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid
- c-AUCB
cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid
- TPAU
1-(1-acetylpiperidin-4-yl)-3-[(4-trifluoromethoxy)phenyl]urea
- AEPU
1-adamantanyl-3-{5-[2-(2-ethoxyethoxy)ethoxy]pentyl]}urea
- APAU
1-(1-acetypiperidin-4-yl)-3-adamantanylurea
- TPAU
1-trifluoromethoxyphenyl-3-(1-acetylpiperidin-4-yl)urea
- Elaidamide
(E)-octadec-9-enamide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Aal YAI, Hammock BD. 3-octylthio-1,1,1-trifluoro- 2-propanone, a high affinity and slow binding inhibitor of juvenile hormone esterase from Trichoplusia ni (hübner) Insect Biochemistry. 1985;15:111–122. [Google Scholar]
- Anspaugh DD, Roe RM. Regulation of JH epoxide hydrolase versus JH esterase activity in the cabbage looper, Trichoplusia ni, by juvenile hormone and xenobiotics. Journal of Insect Physiology. 2005;51:523–535. doi: 10.1016/j.jinsphys.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Arand M, Wagner H, Oesch F. Asp333, Asp495, and His523 form the catalytic triad of rat soluble epoxide hydrolase. The Journal of biological chemistry. 1996;271:4223–4229. doi: 10.1074/jbc.271.8.4223. [DOI] [PubMed] [Google Scholar]
- Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B, Bartholomay L, Bidwell S, Caler E, Camara F, Campbell CL, Campbell KS, Casola C, Castro MT, Chandramouliswaran I, Chapman SB, Christley S, Costas J, Eisenstadt E, Feschotte C, Fraser-Liggett C, Guigo R, Haas B, Hammond M, Hansson BS, Hemingway J, Hill SR, Howarth C, Ignell R, Kennedy RC, Kodira CD, Lobo NF, Mao C, Mayhew G, Michel K, Mori A, Liu N, Naveira H, Nene V, Nguyen N, Pearson MD, Pritham EJ, Puiu D, Qi Y, Ranson H, Ribeiro JM, Roberston HM, Severson DW, Shumway M, Stanke M, Strausberg RL, Sun C, Sutton G, Tu ZJ, Tubio JM, Unger MF, Vanlandingham DL, Vilella AJ, White O, White JR, Wondji CS, Wortman J, Zdobnov EM, Birren B, Christensen BM, Collins FH, Cornel A, Dimopoulos G, Hannick LI, Higgs S, Lanzaro GC, Lawson D, Lee NH, Muskavitch MA, Raikhel AS, Atkinson PW. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science. 2010;330:86–88. doi: 10.1126/science.1191864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, Ramirez JL, Das S, Alvarez K, Arensburger P, Bryant B, Chapman SB, Dong Y, Erickson SM, Karunaratne SH, Kokoza V, Kodira CD, Pignatelli P, Shin SW, Vanlandingham DL, Atkinson PW, Birren B, Christophides GK, Clem RJ, Hemingway J, Higgs S, Megy K, Ranson H, Zdobnov EM, Raikhel AS, Christensen BM, Dimopoulos G, Muskavitch MA. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010;330:88–90. doi: 10.1126/science.1193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beetham JK, Tian TG, Hammock BD. cDNA cloning and expression of a soluble epoxide hydrolase from Human Liver. Archives of Biochemistry and Biophysics. 1993;305:197–201. doi: 10.1006/abbi.1993.1411. [DOI] [PubMed] [Google Scholar]
- Capdevila JH, Falck JR, Estabrook RW. Cytochrome P450 and the arachidonate cascade. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1992;6:731–736. doi: 10.1096/fasebj.6.2.1537463. [DOI] [PubMed] [Google Scholar]
- Casas J, Harshman LG, Hammock BD. Epoxide hydrolase activity on juvenile hormone in Manduca sexta. Insect Biochemistry. 1991;21:17–26. [Google Scholar]
- Dadd RH. Essential fatty acids for the mosquito Culex pipiens. Journal of Nutrition. 1980;110:1152–1160. doi: 10.1093/jn/110.6.1152. [DOI] [PubMed] [Google Scholar]
- Dadd RH. Essential fatty acids for mosquitoes, other insects and vertebrates. 1981 [Google Scholar]
- Dadd RH, Kleinjan JE. Prostaglandin synthetase inhibitors modulate the effect of essential dietary arachidonic acid in the mosquito Culex pipiens. Journal of Insect Physiology. 1984;30:721–728. [Google Scholar]
- Dana AN, Hong YS, Kern MK, Hillenmeyer ME, Harker BW, Lobo NF, Hogan JR, Romans P, Collins FH. Gene expression patterns associated with blood-feeding in the malaria mosquito Anopheles gambiae. BMC Genomics. 2005;6:5. doi: 10.1186/1471-2164-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauterman WC. The role of hydrolases in insecticide metabolism and the toxicological significance of the metabolites. Journal of toxicology. Clinical toxicology. 1982;19:623–635. doi: 10.3109/15563658208990395. [DOI] [PubMed] [Google Scholar]
- Decker M, Adamska M, Cronin A, Di Giallonardo F, Burgener J, Marowsky A, Falck JR, Morisseau C, Hammock BD, Gruzdev A, Zeldin DC, Arand M. EH3 (ABHD9): the first member of a new epoxide hydrolase family with high activity for fatty acid epoxides. Journal of Lipid Research. 2012;53:2038–2045. doi: 10.1194/jlr.M024448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Badillo A, Bolling BG, Perez-Ramirez G, Moore CG, Martinez-Munoz JP, Padilla-Viveros AA, Camacho-Nuez M, Diaz-Perez A, Beaty BJ, Munoz Mde L. The distribution of potential West Nile virus vectors, Culex pipiens pipiens and Culex pipiens quinquefasciatus (Diptera: Culicidae), in Mexico City. Parasit Vectors. 2011;4:70. doi: 10.1186/1756-3305-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falany CN, McQuiddy P, Kasper CB. Structure and organization of the microsomal xenobiotic epoxide hydrolase gene. Journal of Biological Chemistry. 1987;262:5924–5930. [PubMed] [Google Scholar]
- Fulton D, Falck JR, McGiff JC, Carroll MA, Quilley J. A method for the determination of 5,6-EET using the lactone as an intermediate in the formation of the diol. J Lipid Res. 1998;39:1713–1721. [PubMed] [Google Scholar]
- Grant DF, Storms DH, Hammock BD. Molecular cloning and expression of murine liver soluble epoxide hydrolase. Journal of Biological Chemistry. 1993;268:17628–17633. [PubMed] [Google Scholar]
- Harris TR, Aronov PA, Jones PD, Tanaka H, Arand M, Hammock BD. Identification of two epoxide hydrolases in Caenorhabditis elegans that metabolize mammalian lipid signaling molecules. Archives of Biochemistry and Biophysics. 2008;472:139–149. doi: 10.1016/j.abb.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopmann KH, Himo F. Theoretical study of the full reaction mechanism of human soluble epoxide hydrolase. Chemistry-a European Journal. 2006;12:6898–6909. doi: 10.1002/chem.200501519. [DOI] [PubMed] [Google Scholar]
- Jiang H, Anderson GD, McGiff JC. The red blood cell participates in regulation of the circulation by producing and releasing epoxyeicosatrienoic acids. Prostaglandins & Other Lipid Mediators. 2012;98:91–93. doi: 10.1016/j.prostaglandins.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HL, Quilley J, Reddy LM, Falck JR, Wong PYK, McGiff JC. Red blood cells: reservoirs of cis- and trans-epoxyeicosatrienoic acids. Prostaglandins & Other Lipid Mediators. 2005;75:65–78. doi: 10.1016/j.prostaglandins.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Keiser KCL, Brandt KS, Silver GM, Wisnewski N. Cloning, partial purification and in vivo developmental profile of expression of the juvenile hormone epoxide hydrolase of Ctenocephalides felis. Archives of Insect Biochemistry and Physiology. 2002;50:191–206. doi: 10.1002/arch.10044. [DOI] [PubMed] [Google Scholar]
- Khalil SMS, Anspaugh DD, Roe RM. Role of juvenile hormone esterase and epoxide hydrolase in reproduction of the cotton bollworm, Helicoverpa zea. Journal of Insect Physiology. 2006;52:669–678. doi: 10.1016/j.jinsphys.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Knehr M, Thomas H, Arand M, Gebel T, Zeller HD, Oesch F. Isolation and characterization of a cDNA encoding rat liver cytosolic epoxide hydrolase and its functional expression in Escherichia coli. Journal of Biological Chemistry. 1993;268:17623–17627. [PubMed] [Google Scholar]
- Morisseau C. Measurement of soluble epoxide hydrolase (sEH) activity. Current Protocols in Toxicology. 2007;33 doi: 10.1002/0471140856.tx0423s33. 34.23:34.23.31–34.23.18. [DOI] [PubMed] [Google Scholar]
- Morisseau C. Role of epoxide hydrolases in lipid metabolism. Biochimie. 2013;95:91–95. doi: 10.1016/j.biochi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annual review of pharmacology and toxicology. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Hammock BD. Gerry Brooks and epoxide hydrolases: four decades to a pharmaceutical. Pest Manag Sci. 2008;64:594–609. doi: 10.1002/ps.1583. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annual review of pharmacology and toxicology. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin CA. Adaptive relationships of epoxide hydrolase in herbivorous arthropods. Journal of chemical ecology. 1988;14:1867–1888. doi: 10.1007/BF01013483. [DOI] [PubMed] [Google Scholar]
- Pakpour N, Akman-Anderson L, Vodovotz Y, Luckhart S. The effects of ingested mammalian blood factors on vector arthropod immunity and physiology. Microbes and Infection. 2013;15:243–254. doi: 10.1016/j.micinf.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BPN, Labbe P, Corbel V. Culex genome is not just another genome for comparative genomics. Parasites & Vectors. 2012;5 doi: 10.1186/1756-3305-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders HR, Evans AM, Ross LS, Gill SS. Blood meal induces global changes in midgut gene expression in the disease vector, Aedes aegypti. Insect Biochem Mol Biol. 2003;33:1105–1122. doi: 10.1016/s0965-1748(03)00124-3. [DOI] [PubMed] [Google Scholar]
- Seino A, Ogura T, Tsubota T, Shimomura M, Nakakura T, Tan A, Mita K, Shinoda T, Nakagawa Y, Shiotsuki T. Characterization of juvenile hormone epoxide hydrolase and related genes in the larval development of the silkworm Bombyx mori. Bioscience Biotechnology and Biochemistry. 2010;74:1421–1429. doi: 10.1271/bbb.100104. [DOI] [PubMed] [Google Scholar]
- Severson TF, Goodrow MH, Morisseau C, Dowdy DL, Hammock BD. Urea and amide-based inhibitors of the juvenile hormone epoxide hydrolase of the tobacco hornworm (Manduca sexta: Sphingidae) Insect Biochemistry and Molecular Biology. 2002;32:1741–1756. doi: 10.1016/s0965-1748(02)00115-7. [DOI] [PubMed] [Google Scholar]
- Shen HC, Hammock BD. Discovery of inhibitors of soluble epoxide hydrolase: A target with multiple potential therapeutic indications. Journal of Medicinal Chemistry. 2011;55:1789–1808. doi: 10.1021/jm201468j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoda RC, Demierre A, McBride OW, Gonzalez FJ, Meyer UA. Human microsomal xenobiotic epoxide hydrolase. Complementary DNA sequence, complementary DNA-directed expression in COS-1 cells, and chromosomal localization. Journal of Biological Chemistry. 1988;263:1549–1554. [PubMed] [Google Scholar]
- Stanley D. Prostaglandins and other eicosanoids in insects: Biological significance. Annual Review of Entomology. 2006:25–44. doi: 10.1146/annurev.ento.51.110104.151021. [DOI] [PubMed] [Google Scholar]
- Stanley D, Kim Y. Eicosanoid signaling in insects: from discovery to plant protection. Critical Reviews in Plant Sciences. 2014;33:20–63. [Google Scholar]
- Stanley DW, Miller JS. Eicosanoid actions in insect cellular immune functions. Entomologia Experimentalis Et Applicata. 2006;119:1–13. [Google Scholar]
- Stanley-Samuelson DW, Jurenka RA, Cripps C, Blomquist GJ, Derenobales M. Fatty acids in insects - composition, metabolism, and biological significance. Archives of Insect Biochemistry and Physiology. 1988;9:1–33. [Google Scholar]
- Taniai K, Inceoglu AB, Yukuhiro K, Hammock BD. Characterization and cDNA cloning of a clofibrate-inducible microsomal epoxide hydrolase in Drosophila melanogaster. European Journal of Biochemistry. 2003;270:4696–4705. doi: 10.1046/j.1432-1033.2003.03868.x. [DOI] [PubMed] [Google Scholar]
- Tapiero H, Nguyen Ba G, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomedicine & Pharmacotherapy. 2002;56:215–222. doi: 10.1016/s0753-3322(02)00193-2. [DOI] [PubMed] [Google Scholar]
- Touhara K, Soroker V, Prestwich GD. Photoaffinity-labeling of juvenile hormone epoxide hydrolase and JH-binding proteins during ovarian and egg development in Manduca sexta. Insect Biochemistry and Molecular Biology. 1994;24:633–640. [Google Scholar]
- Tsubota T, Nakakura T, Shiotsuki T. Molecular characterization and enzymatic analysis of juvenile hormone epoxide hydrolase genes in the red flour beetle Tribolium castaneum. Insect Molecular Biology. 2010;19:399–408. doi: 10.1111/j.1365-2583.2010.01001.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Morisseau C, Hammock BD. Expression and characterization of an epoxide hydrolase from Anopheles gambiae with high activity on epoxy fatty acids. Insect Biochemistry and Molecular Biology. 2014;54:42–52. doi: 10.1016/j.ibmb.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Analytical Chemistry. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZG, Xu FY, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circulation Research. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. Journal of Biological Chemistry. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, Capdevila JH. Regiofacial and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. Journal of Biological Chemistry. 1993;268:6402–6407. [PubMed] [Google Scholar]
- Zhang QR, Xu WH, Chen FS, Li S. Molecular and biochemical characterization of juvenile hormone epoxide hydrolase from the silkworm, Bombyx mori. Insect Biochemistry and Molecular Biology. 2005;35:153–164. doi: 10.1016/j.ibmb.2004.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.