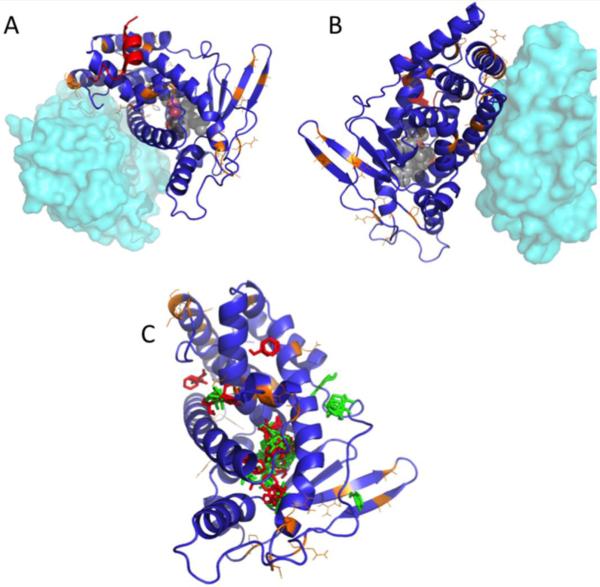
Figure 4. Homology model for pbPXR based on multiple hPXR structures showing residue substitutions between human and polar bear.
(A and B) Positions of residues differing between hPXR and pbPXR are shown in orange. Also shown is the relative position of the heterodimerization partner, RXR, from PDB 4J5W in cyan, and the position of the coactivator protein SRC1 from PDB 2O9I in red. (C) Positions of solvent-mapped small molecule clusters from FTMAP are shown in red (pbPXR) and green (hPXR). Note that most clusters fall into the known ligand binding pocket, but there are significant clusters found outside the pocket, which differ between hPXR and pbPXR.