Figure 5. Pharmacophores generated from the best-binding ligands for hPXR (A) and pbPXR (B).
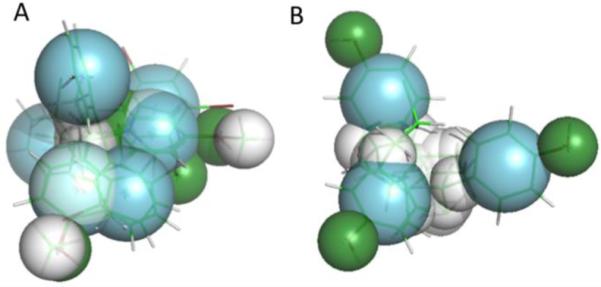
Aromatic overlaps are shown in blue, hydrophobic regions in white, and hydrophilic donor/acceptors are shown in green. Note the pyramidal shape for both pharmacophores, fitting the shape of the known PXR binding pocket. However, the human pharmacophore differs in the positioning of aromatic residues at one pyramid apex, in constrast to the pbPXR. Direct residue substitutions are not observed in the binding pocket, suggesting that longer range differences in the tertiary structure e.g. via A281T may play a role in the different agonist profiles between human and polar bear PXR.
