Figure 2. Model of TFEB regulation and function during starvation.
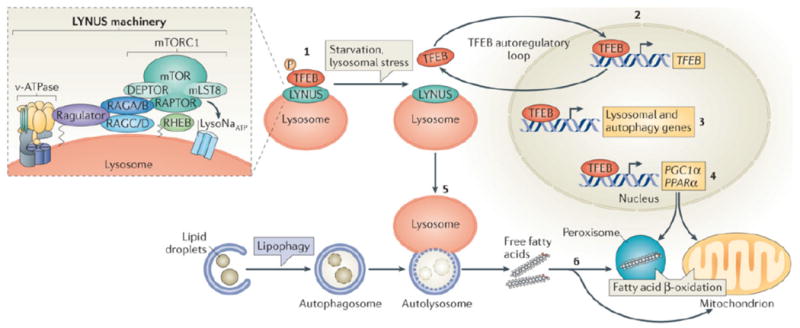
This model illustrates how transcription factor EB (TFEB) is induced by starvation and mediates the starvation response by regulating lipid catabolism. In the presence of adequate nutrition TFEB interacts with the lysosome nutrient sensing (LYNUS) machinery, which senses lysosomal nutrient levels via the vATPase complex, and is phosphorylated by mTORC1 on the lysosomal surface (1). This keeps TFEB inactive by cytosolic sequestration. During starvation mTORC1 is released from the LYNUS machinery and becomes inactive. Thus, TFEB can no longer be phosphorylated by mTORC1 and it translocates to the nucleus where it induces its own transcription (2). Therefore, starvation regulates TFEB activity through a dual mechanism that involves a post-translational modification (that is, phosphorylation) and a transcriptional autoregulatory loop. Once in the nucleus, TFEB regulates the expression of genes involved in the lysosomal–autophagy pathway (3). and of PGC1α–PPARα target genes (4). In this way TFEB controls the starvation response by activating both macrolipophagy (5) and fatty-acid oxidation (6).
The insert shows the main components of the LYNUS machinery. The mTORC1 complex, which includes regulatory proteins associated with mTOR, such as RAPTOR, LST8, and DEPTOR211, physically interacts with the RAG GTPases on the lysosomal surface and it is activated by them212. A complex known as the Ragulator mediates the activation and docking of RAGS to the lysosomal membrane97, 213 and the small GTPase RHEB is also involved in the growth factor-mediated activation of mTORC1214, 215. The vATPase complex is involved in amino acid sensing and it mediates amino acid-sensitive interactions between Rags and Ragulator, which is the initial step in lysosomal signaling104. The ATP-sensitive Na+ channel lysoNaATP, which is comprised of the subunits TPC1 and TPC2, is located on the lysosomal membrane and it has recently been shown to interact with mTORC1 and participates in nutrient sensing106 The nature of interaction between lysoNaATP and mTORC1 is unknown but seems to be independent form other components of the LYNUS machinery and the transcription factor EB (TFEB) and its interacting proteins (see text).
