Abstract
Cutaneous lupus erythematosus (CLE) is a chronic inflammatory autoimmune skin disease. Evidence-based therapy for CLE is lacking in the most part. Intravenous immunoglobulin (IVIg) is being increasingly utilized as off-label therapy for a variety of autoimmune and inflammatory conditions, especially in dermatology. The usefulness of IVIg in CLE is not well established. The goal of the present study was to obtain the proof-of-concept evidence that IVIg can control acute CLE and thus replace current systemic immunosuppressive therapy that causes severe side effects and adverse reactions. Sixteen patients who tried and failed various systemic treatments for CLE were screened and consented to use IVIg as a monotherapy. The IVIg was administered at 500 mg/kg/day on 4 consecutive days up to a total of 2 g/kg/month for 3 months, and the subjects were monitored for additional 6 months off any drug for a possible relapse. The cumulative results revealed an overall improvement, as evinced by a decrease of both objective and subjective measures of disease activity. The most sensitive and specific objective and subjective instruments for assessment of the therapeutic effect of IVIg were CLASI-A (Cutaneous Lupus Erythematosus Disease Area and Severity Index) measuring disease activity and Skindex-29 scores, respectively. The CLASI-A score dropped down from the initial value taken as 100%, and remained in the range of approximately 70% until the last visit. Three patients (18.8%) had a temporary flare of CLE symptoms but recovered within a month from the relapse. No serious side effects and adverse reactions occurred. Thus, IVIg monotherapy in CLE allowed to achieve: i) rapid and persistent decreased in disease activity; ii) steady improvement of patients’ quality of life assessed by Skindex-29; iii) low relapse rate; and iv) mild nature and short duration of relapses. Since healing was maintained for months after IVIg treatment, it is possible that the IVIgtriggered molecular events mediating the therapeutic action of IVIg that continued to unfold after the end of therapy.
Key words: cutaneous lupus erythematosus, IVIg, case-series, CLASI, Skindex-29
Introduction
Cutaneous lupus erythematosus (CLE) is a chronic inflammatory autoimmune skin disease that may cause permanent scarring. Discoid lupus erythematosus, subacute cutaneous lupus erythematosus, lupus panniculitis and lupus erythematosus tumidus all fall into the category of CLE. Face and scalp are most commonly affected by scarring in the discoid form of CLE. The etiology and pathogenesis of CLE are multifactorial with genetic and environmental factors playing a role. Photosensitivity, polymorphisms of the major histocompatibility complex leading to increased immune response to self-antigens, deficiencies of complement components, gender, and autoantibodies are all thought to play a role. Anti-Ro, anti-La, anti-dsDNA and antinucleosome antibodies are thought to play a role in skin disease by increased skin cell apoptosis. Dysregulation of T cells may also play a role in the pathogenesis of CLE.1,2
Evidence-based therapy for CLE is lacking. Administration of systemic immunosuppressive and anti-inflammatory agents, such as corticosteroids, hydroxychloroquine, mepacrine, methotrexate, mycophenolate mofetil, cyclophosphamide and azathioprine, in many cases, leads to remission. Nevertheless, many patients suffer from resistant cutaneous lesions despite therapy. Alternative systemic (dapsone, thalidomide, retinoids, cyclosporine) and topical agents (thalidomide, intralesional steroids, retinoids, tacrolimus ointment) as well as laser therapy, phototherapy, photopheresis, and cryotherapy are used for resistant cutaneous lesions, but a positive outcome is not guaranteed.3-5
Intravenous immunoglobulin (IVIg) is a fractioned blood product consisting of pooled, polyvalent, IgG antibody extracted from the plasma of over 10,000 blood donors per batch. Historically, it was used to treat primary and secondary immune deficiencies, however, its use has expanded tremendously over the past several decades. Today, it is being increasingly utilized as off-label therapy for a variety of autoimmune and inflammatory conditions, especially in dermatology. IVIg exhibits several effects with the most well described being: i) complement blockade and degradation; ii) neonatal Fc receptor saturation; iii) induction of immunomodulatory Fc receptors; iv) inhibition of B cells; and v) altering T cell function, cytokine production and migration.6 The increasing use of IVIg has been associated with further understanding of its mechanisms of action, clinical manipulation and associated side effects, as well as the introduction of improved or new types of IVIg products. Ongoing research continues to elaborate and identify novel mechanisms. One area in which IVIg is continuing to make dramatic improvements is the ability to treat derangements of immunity in immune-mediated diseases.7
Although the usefulness of IVIg in CLE is not well established, IVIg has been used to successfully treat CLE, either as monotherapy or in conjunction with an immunosuppressant.8-13
The first report in 1997 described 7 female patients treated with IVIg at a dosage of 300 mg/kg per day for 5 consecutive days each month for a 12-month period.14 Although some clinical symptoms, such as asthenia, arthromyalgia, and fever, disappeared in some patients and immunologic parameters improved, IVIg was not able to improve the cutaneous manifestations. Moreover, skin lesions of CLE worsened. The vast majority of subsequent reports, however, demonstrated an overall good efficacy of IVIg in resistant cases.8,11-13,15 For instance, in the responding patients, the duration of the remission ranged from 2 months to more than 1 year, suggesting that repeated pulses of IVIg could be used as maintenance therapy. For example, IVIg showed beneficial effects in a 2-day course (1 g/kg per day) every 4 weeks in 5 patients with refractory extensive long-lasting discoid CLE with major face involvement. Relapses occurred 2 to 10 months after IVIg withdrawal and skin lesions were controlled again with IVIg in one patient with discoid CLE. In another case report, one patient with resistant CLE was treated with IVIg (2 g/kg per month) and showed dramatic improvement within 3 months and almost complete resolution after 6 months. Three patients with CLE unresponsive to previous therapy were treated with IVIg, which achieved good response of their skin lesions. Twelve patients with histologically confirmed CLE were given IVIg with starting doses of 1 g/kg per day on 2 consecutive days, followed by 400 mg/kg per month for 6 months or until disease resolution. Five patients had complete or near complete clearing of their skin disease (>75% clearing), two had partial improvement (~50% clearing), and three had limited response (<50% clearing). Others reported a female patient with highly recalcitrant CLE who responded very well to treatment with IVIg (1 g/kg per day on 3 consecutive days per month). Amelioration was observed after the first cycle followed by an almost complete clearing. More recently, a case of CLE successfully treated with IVIg was reported after other therapy modalities failed. Thus, the available data strongly suggest that patients with CLE may benefit from a therapy with IVIg. However, a recommended or defined protocol for patients with CLE does not exist.
The goal of the present study was to obtain the proof-of-concept evidence that IVIg can control acute CLE and thus replace current systemic immunosuppressive therapy that causes severe side effects and adverse reactions. From the review of literature, we postulated that: i) the beneficial effects of IVIg for patients with CLE should be prompt, with marked improvement within a few weeks; and ii) clinical improvement should last several weeks after the last infusion.
Materials and Methods
For this small scale exploratory consecutive case-series type of study, subjects were recruited from an outpatient CLE patient population treated at the University of California, Irvine. Sixteen patients who received a standard therapy without a therapeutic response for a minimum of one month were screened and consented to use IVIg as a monotherapy. Our patients have tried and failed various systemic treatments for CLE, such as prednisone, plaquenil and dapsone. Topical treatment with clobetasol or tacrolimus, which was not effective either, was stopped at the beginning of IVIg therapy.
Inclusion criteria: males and females ≥18 years of age; active CLE (any subtype) established by standard clinical and histo- and immunopathological criteria; understands and follows directions.
Exclusion criteria: subjects <18 years of age; cannot understand or follow directions; a pregnant, planning to get pregnant or breast feeding female; a female of child-bearing potential and unwilling to use a form of highly effective birth control; has one or more of the following problems: IgA deficiency, HIV infection, organ transplant, severe anemia, jaundice, major organ failure, creatinine >1.5 mg/dL or hemoglobin <11.0 g/dL.
All enrolled subjects received IVIg treatment following the protocol that proved to be efficacious in the treatment of patients with autoimmune blistering diseases as well as previously treated patients with CLE. The IVIg (GAMUNEX-C, Grifols, Los Angeles, CA, USA) was administered at 500 mg/kg/day on 4 consecutive days up to a total of 2 g/kg/month for 3 months in the Institute for Clinical and Translational Science at University of California, Irvine. After 3 months of treatment, IVIg was discontinued and the subjects were monitored for additional 6 months off any drug for a possible relapse.
Upon entry into the study, the study subject’s severity of CLE was evaluated using the following instruments: Physician’s Subjective Assessment of Severity (PSAS; mild, moderate or severe), Physician’s Subjective Assessment of Improvement (PSAI; improved, unchanged or worse), and Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) measuring both the activity of the disease (CLASI-A) and the damage done by the disease (CLASI-D). The higher scores indicated more severe skin disease.16 The subjects also evaluated their skin-specific quality of life with the Skindex-29 – the questionnaire consisting of 29 items used to calculate three sub-scales: symptoms (pain, itch, burning, sensitivity), emotions (depression, anxiety, embarrassment, anger) and functioning (sleep, relationships with others).16 All assessments were repeated at all study visits.
Results
We have enrolled and treated 15 female and 1 male CLE patients aged 19-70 years with 3 monthly cycles of IVIg monotherapy. The cumulative results of assessment of clinical efficacy of IVIg in the enrolled patients revealed an overall improvement, as evinced by a decrease of both objective and subjective measures of disease activity. The most sensitive and specific objective and subjective instruments for assessment of the therapeutic effect of IVIg were CLASI-A and Skindex-29 scores, respectively (Table 1). The individual variations of CLASI-A and Skindex-29 scores are shown in Figure 1. All other measured parameters, CLASI-D, PSAS and PSAI, did not correlate with clinical improvement. As seen in Table 1, on the 7th visit after starting IVIg treatment, the CLASI-A score dropped down to 68% from the initial value taken as 100%, and remained in the range of approximately 70% until the last visit. Skindex-29 was found to be an informative subjective measure, showing a drop to 79% from the initial 100% on the 6th visit (Table 1). After that, Skindex-29 has increased again.
Table 1.
The clinical response scores (%) in enrolled cutaneous lupus erythematosus patients treated by intravenous immunoglobulin monotherapy.
| Clinical evaluation test | Initial visit (n=16) | 1st visit (n=14) | 2nd visit (n=14) | 3rd visit (n=12) | 4th visit (n=9) | 5th visit (n=6) | 6th visit (n=6) | 7thvisit (n=6) | 8th visit (n=5) | 9th visit (n=6) |
|---|---|---|---|---|---|---|---|---|---|---|
| CLASI-A | 100 | 94 | 84 | 94 | 86 | 85 | 72 | 68 | 81 | 71 |
| CLASI-D | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| PSAS | 100 | 100 | 98 | 100 | 97 | 92 | 92 | 92 | 90 | 92 |
| PSAI | 100 | 100 | 100 | 136 | 104 | 105 | 117 | 133 | 15 | 100 |
| Skindex-29 | 100 | 97 | 94 | 92 | 90 | 81 | 79 | 83 | 99 | 92 |
CLASI, cutaneous lupus erythematosus disease area and severity index; PSAS, physician’s subjective assessment of severity; PSAI, physician’s subjective assessment of improvement. Results are expressed as a relative mean value determined for the entire group of cutaneous lupus erythematosus on a particular visit number, compared to the pre-treatment value for the corresponding test, taken as 100%. Number of patients per each particular time-point (i.e., visit number), for who the mean value was calculated.
Figure 1.
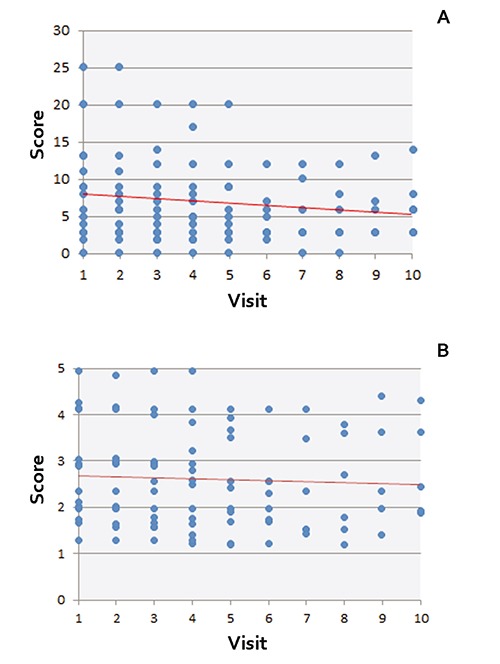
Individual cutaneous lupus erythematosus disease area and severity index-total activity score (A) and Skindex-29 (B) values in enrolled cutaneous lupus erythematosus patients. The vertical axis shows the score for each patient (blue dots), and the horizontal axis indicates the visit number, starting from initial visit before starting immunoglobulin monotherapy, i.e., visit #1. The red line depicts the best fit line relative to all data points. Note: Values for some individual patient overlay. Visit 1: screening; Visit 2: baseline; Visit 3: month 1; Visit 4: month 2; Visit 5: month 3; Visit 6: month 4; Visit 7: month 5; Visit 8: month 6; Visit 9: month 7; Visit 10: month 8.
First dose response was indicated by a steady decrease in CLASI-A between the baseline and the 1st month after starting IVIg treatment. Five patients had a decrease in CLASI-A that persisted for the rest of the study. Three patients (18.8%) had a temporary flare of CLE symptoms categorized as a relapse of the disease. All patients recovered within a month from the relapse. In one patient, the CLASI-A score increased from 7 to 10 but then dropped to 8. In a second patient, the CLASI-A score increased from 2 to 5 and then decreased back to 2. In a third patient, the CLASI-A score increased from 14 to 17 but then dropped to 9.
No serious side effects and adverse reactions occurred. Two patients developed mild anemia, 1 mild neutropenia, 5 headache, 1 back aches, 1 high blood pressure, 2 fever, 1 gastro-intestinal upset, 2 nausea/vomiting, and 1 herpes-associated erythema multiforme. These are commonly seen in patients with various medical conditions receiving IVIg therapy.
Discussion
The results of this proof-of-concept study of clinical efficacy of a short-term IVIg monotherapy in CLE produced very promising results, as evidenced by: i) rapid and persistent decreased in disease activity measured by CLASI-A scores; ii) steady improvement of patients’ quality of life assessed by Skindex-29; iii) low relapse rate; and iv) mild nature and short duration of relapses.
Since healing was maintained for months after IVIg treatment, it is possible that the IVI-gtriggered molecular events mediating the therapeutic action of IVIg that continued to unfold after the end of therapy. The results of this study thus provide the basis for hypotheses for the future multicenter randomized controlled studies to identify which CLE subsets will benefit the most and which protocol will provide the optimal clinical outcome.
The obtained results indicated that a minimum of 3 treatment cycles with IVIg at 2 g/kg/month were needed to achieve clinical response. However, the fact that both CLASI-A and Skindex-29 scores showed a tendency to increase after reaching the minimal values strongly suggests that more cycles of IVIg monotherapy are required to achieve a stable clinical remission off any drugs. This would be in keeping with duration of IVIg therapy required to achieve stable remission in patients with autoimmune blistering dermatoses. For examples, an average number of IVIg cycles (i.e., months) of treatment of patients with pemphigus and pemphigoid is equal to 38.6.17 Thus, while the use of IVIg allows achievement of rapid remission of CLE, the maintenance therapy with repeated monthly pulses of IVIg for an extended period of time should maintain the disease in remission. Future clinical trials should determine the optimal number of cycles of treatment for patients with distinct CLE forms by IVIg monotherapy.
In this study, CLASI-A was the most sensitive measure of treatment efficacy. The obtained results are comparable to those reported by the others who tested the efficacy of CLE treatment by pulsed dye laser treatment or Vitamin D.18,19 Overall, CLASI score measurement shows good content validity, addresses the most relevant aspects of all subsets of CLE, including individual lesions localized and generalized CLE, and also has good inter-rater and intra-rater reliability when used by either dermatologists or rheumatologists.16,20-26 In marked contrast, CLASI-D remained unchanged for all but 2 patients, as could be expected for such a short-term study, given the irreversible nature of skin damage in CLE, e.g., scars. The CLASI-D result is comparable to other CLE studies that have been shown to treat CLE.
The relapses in the three of our patients, defined as an increase in CLASI-A score, were mild, because within one month, their CLASI-A became comparable to or even lower than that prior to the relapse. In our patients, the relapse rate was much lower, compared to that reported in the literature for CLE patients. For comparison, in other clinical trials the relapse rate was reported at the range of 70-80%.27-29
Conclusions
We can conclude that: i) IVIg monotherapy clearly alleviated symptoms in patients with different clinical forms of CLE resistant to standard treatments, which should justify a multi-center clinical trial on a larger number of patients to ultimately establish the efficacy of IVIg in each clinical form of CLE; ii) three monthly cycles of 2 g/kg/month of IVIg were sufficient to achieve a stable remission, but the symptoms may return, indicating the need for a more extended duration IVIg monotherapy of CLE, by analogy with other mucocutaneous autoimmune diseases; iii) CLASI-A and Skindex-29 appeared to be the most sensitive objective and subjective, respectively, instruments for analysis of clinical efficacy of IVIg in patients with CLE, and, therefore, should be employed for assessment of treatment efficacy in future clinical trials of IVIg in CLE; iv) treatment with IVIG dramatically decreases the frequency and severity of relapses, compared to some previously tested therapies CLE patients.
Acknowledgements
This work was supported by a research grant from Grifols and a grant-in-aid from Institute for Clinical and Translational Science at University of California, Irvine.
References
- 1.Yu C, Chang C, Zhang J. Immunologic and genetic considerations of cutaneous lupus erythematosus: a comprehensive review. J Autoimmun 2013;41:34-45. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn A, Wenzel J, Weyd H. Photosensitivity, apoptosis, and cytokines in the pathogenesis of lupus erythematosus: a critical review. Clin Rev Allergy Immunol 2014;47:148-62. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part II. J Am Acad Dermatol 2011;65:e195-213. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol 2011;65:e179-93. [DOI] [PubMed] [Google Scholar]
- 5.Pelle MT. Issues and advances in the management and pathogenesis of cutaneous lupus erythematosus. Adv Dermatol 2006;22:55-65. [DOI] [PubMed] [Google Scholar]
- 6.Luzi G, Bongiorno F, Paparo Barbaro S, Bruno G. Intravenous IgG: biological modulating molecules. J Biol Regul Homeost Agents 2009;23:1-9. [PubMed] [Google Scholar]
- 7.Gurcan HM, Ahmed AR. Efficacy of various intravenous immunoglobulin therapy protocols in autoimmune and chronic inflammatory disorders. Ann Pharmacother 2007;41:812-23. [DOI] [PubMed] [Google Scholar]
- 8.Goodfield M, Davison K, Bowden K. Intravenous immunoglobulin (IVIg) for therapy-resistant cutaneous lupus erythematosus (LE). J Dermatolog Treat 2004;15:46-50. [DOI] [PubMed] [Google Scholar]
- 9.Genereau T, Chosidow O, Danel C, et al. High-dose intravenous immunoglobulin in cutaneous lupus erythematosus. Arch Dermatol 1999;135:1124-5. [DOI] [PubMed] [Google Scholar]
- 10.Kreuter A, Gaifullina R, Tigges C, et al. Lupus erythematosus tumidus: response to antimalarial treatment in 36 patients with emphasis on smoking. Arch Dermatol 2009;145:244-8. [DOI] [PubMed] [Google Scholar]
- 11.Kreuter A, Hyun J, Altmeyer P, Gambichler T. Intravenous immunoglobulin for recalcitrant subacute cutaneous lupus erythematosus. Acta Derm Venereol 2005;85:545-7. [DOI] [PubMed] [Google Scholar]
- 12.Espirito Santo J, Gomes MF, Gomes MJ, et al. Intravenous immunoglobulin in lupus panniculitis. Clin Rev Allergy Immunol 2010;38:307-18. [DOI] [PubMed] [Google Scholar]
- 13.Lampropoulos CE, Hughes GR, DP DC. Intravenous immunoglobulin in the treatment of resistant subacute cutaneous lupus erythematosus: a possible alternative. Clin Rheumatol 2007;26:981-3. [DOI] [PubMed] [Google Scholar]
- 14.De Pita O, Bellucci AM, Ruffelli M, et al. Intravenous immunoglobulin therapy is not able to efficiently control cutaneous manifestations in patients with lupus erythematosus. Lupus 1997;6:415-7. [DOI] [PubMed] [Google Scholar]
- 15.Levy Y, Sherer Y, Ahmed A, et al. A study of 20 SLE patients with intravenous immunoglobulin-clinical and serologic response. Lupus 1999;8:705-12. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Moghadam-Kia S, LoMonico J, et al. Development of the CLASI as a tool to measure disease severity and responsiveness to therapy in cutaneous lupus erythematosus. Arch Dermatol 2011;147:203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seidling V, Hoffmann JH, Enk AH, Hadaschik EN. Analysis of high-dose intravenous immunoglobulin therapy in 16 patients with refractory autoimmune blistering skin disease: high efficacy and no serious adverse events. Acta Derm Venereol 2013;93:346-9. [DOI] [PubMed] [Google Scholar]
- 18.Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol 2009;60:626-32. [DOI] [PubMed] [Google Scholar]
- 19.Cutillas-Marco E, Marquina-Vila A, Grant W, et al. Vitamin D and cutaneous lupus erythematosus: effect of vitamin D replacement on disease severity. Lupus 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Albrecht J, Taylor L, Berlin JA, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol 2005;125:889-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albrecht J, Werth VP. Development of the CLASI as an outcome instrument for cutaneous lupus erythematosus. Dermatol Ther 2007;20:93-101. [DOI] [PubMed] [Google Scholar]
- 22.Bonilla-Martinez ZL, Albrecht J, Troxel AB, et al. The cutaneous lupus erythematosus disease area and severity index: a responsive instrument to measure activity and damage in patients with cutaneous lupus erythematosus. Arch Dermatol 2008;144:173-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krathen MS, Dunham J, Gaines E, et al. the cutaneous lupus erythematosus disease activity and severity index: expansion for rheumatology and dermatology. Arthritis Rheum 2008;59:338-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salphale P, Danda D, Chandrashekar L, et al. The study of cutaneous lupus erythematosus disease area and severity index in Indian patients with systemic lupus erythematosus. Lupus 2011;20:1510-7. [DOI] [PubMed] [Google Scholar]
- 25.Piette EW, Foering KP, Chang AY, et al. Impact of smoking in cutaneous lupus erythematosus. Arch Dermatol 2012;148:317-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braunstein I, Klein R, Okawa J, Werth VP. The IFN-regulated gene signature is elevated in SCLE and DLE and correlates with CLASI score. Br J Dermatol 2012;166:971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cortes-Hernandez J, Avila G, Vilardell-Tarres M, Ordi-Ros J. Efficacy and safety of lenalidomide for refractory cutaneous lupus erythematosus. Arthritis Res Ther 2012;14:R265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortes-Hernandez J, Torres-Salido M, Castro-Marrero J, et al. Thalidomide in the treatment of refractory cutaneous lupus erythematosus: prognostic factors of clinical outcome. Br J Dermatol 2012;166:616-23. [DOI] [PubMed] [Google Scholar]
- 29.Coelho A, Souto MI, Cardoso CR, et al. Long term thalidomide use in refractory cutaneous lesions of lupus erythematosus: a 65 series of Brazilian patients. Lupus 2005;14:434-9. [DOI] [PubMed] [Google Scholar]


