Abstract
Background and objective
Chronic obstructive pulmonary disease (COPD) represents an increasing healthcare concern as a leading cause of morbidity and mortality worldwide. Our objective was to predict the outcome of COPD patients associated with multiple organ dysfunction syndrome (MODS) by scoring models.
Methods
A retrospective study was performed on severe COPD patients within 24 hours of the onset of MODS. The Acute Physiology and Chronic Health Evaluation (APACHE) II, APACHE III, Multiple Organ Dysfunction Score (MODS), Simplified Acute Physiology Score II (SAPS II), and Sepsis-related Organ Failure Assessment (SOFA) scores were calculated for patients.
Results
A total of 153 elderly patients were recruited. Compared to 30-day survivors, the number of failing organs and all of the scoring models were significantly higher in 30-day non-survivors. The SOFA showed the highest sensitivity and area under the curve (AUC) for predicting the prognosis of patients with MODS induced by acute exacerbation of COPD. The results of logistic regression indicated that factors that were correlated with the prognosis of COPD included the exacerbation history, SOFA score, number of failing organs, and duration of ICU stay. The value of exacerbation frequency for predicting the outcome of COPD was excellent (AUC: 0.892), with a sensitivity of 0.851 and a specificity of 0.797.
Conclusions
The SOFA score, determined at the onset of MODS in elderly patients with COPD, was a reliable predictor of the prognosis. The exacerbation frequency, number of failing organs, and the SOFA score were risk factors of a poor prognosis, and the exacerbation frequency could also effectively predict the outcome of COPD.
Keywords: Acute exacerbation (AE), chronic obstructive pulmonary disease (COPD), multiple organ dysfunction syndrome (MODS), prognosis
Introduction
Chronic obstructive pulmonary disease (COPD) is a prevalent disease and a major cause of morbidity in elderly patients worldwide (1,2). Severe exacerbation of COPD has been associated with increased mortality (3), which has been attributed to complex underlying diseases that are more likely to progress into multiple organ dysfunction syndrome (MODS) due to their association with inflammatory mediators (4). In 2008, COPD was the fourth leading cause of death in urban areas and the third leading cause in rural areas in China (5). Results are consistent with the American epidemic, and what is worse, COPD will be the third leading cause of death by 2020 in America (6). It has been reported that the total expense of COPD was $1,963.8 per patient, which accounted for 40% of the average family’s total income ($4,849.8) in China in 2006 (5). Presently, COPD has aroused considerable concern in the medical and scientific communities due to its poor prognosis and the growing, substantial burden the disease imposes on healthcare systems (7).
The MODS is characterized by more than one organ system failing, especially during critical illness (8). Therefore, it is important to assess the severity and predict outcomes of elderly COPD patients (age ≥60 years old) with MODS to improve the therapeutic treatments and prognosis of this disease.
Scoring models have been widely utilised in the intensive care unit (ICU) setting and provide physicians with objective information to help inform decisions related to the treatment and prognosis of COPD. In the present study, we compare the predictive value of different scoring models for critically ill patients with COPD, including the Acute Physiology and Chronic Health Evaluation scores [APACHE II (9) and APACHE III (10)], Multiple Organ Dysfunction Score (MODS) (11), Simplified Acute Physiology Score II (SAPS II) (12), and Sepsis-related Organ Failure Assessment (SOFA) Score (13), which have been evaluated and validated in many centres and are widely used by most ICUs to predict clinical outcomes.
Methods
Patient recruitment
We performed a retrospective analysis of 153 patients, aged 60 years or older (http://www.who.int), who were admitted to the Respiratory ICU and Emergency ICU of the Chinese People’s Liberation Army (PLA) General Hospital between January 2008 and June 2012.
Patients who met the following conditions were included in the study: (I) on admission to the hospital, a diagnosis of acute exacerbation (AE) of COPD (AECOPD), without any organ dysfunction, was made based on clinical symptoms and the GOLD guidelines (14); (II) the patient developed MODS during hospitalisation, the definition of which was proposed by the American College of Chest Physicians/Society of Critical Care Medicine (ACCP/SCCM) (15); and (III) the patient’s prognosis was clear based on 30-day outcome (survivors or non-survivors). Patients with the following conditions were excluded from this study: (I) acute respiratory distress syndrome; (II) massive pulmonary embolism; (III) heart failure or cancer; or (IV) respiratory failure due to reasons other than COPD.
All of the patients signed written informed consent forms. The present study was approved by the Clinical Ethics Committee of the Chinese PLA General Hospital.
Definition of acute exacerbation (AE) of COPD
AECOPD is an acute event characterised by an increase in the severity of the patient’s respiratory symptoms (increased sputum volume, acutely worsening dyspnea, and the presence of purulent sputum) that is beyond normal day-to-day variations, and leads to a change in treatment (14,16,17).
Data extraction
The starting point of this study was the day when a patient developed MODS associated with the AECOPD described at the time of hospitalisation. Clinical data from 153 elderly patients were collected and analysed retrospectively, including basic information (e.g., age, gender, and co-morbid diseases), exacerbation history in the preceding year, the number of failing organs, important vital signs (e.g., body temperature, breathing rate, heart rate, and blood pressure) laboratory tests (e.g., blood tests such as white blood cell and platelet counts), blood biochemistry (e.g., bilirubin, creatinine, and electrolytes) within 24 hours of the onset of MODS, duration of the ICU stay, and the 30-day outcome. The APACHE II, APACHE III, MODS, SAPS II and SOFA scores were calculated.
Statistical analysis
The SPSS Statistics 17.0 software (SPSS Inc. Chicago, Illinois, USA) for Windows was used for data analysis. Data were expressed as the mean ± standard deviation (SD) (normal distribution) or as the median (interquartile range for non-normal distributions) for continuous variables and as percentages for categorical variables. Student’s t-test was used to compare normally distributed data between two groups. An analysis of variance was employed to compare multiple groups, and the Kruskal-Wallis rank sum test was used to compare two groups with non-normal data. The qualitative results were evaluated using the chi-square test. The predictive values of the scoring systems were analysed using a receiver operating characteristic (ROC) curve, and the sensitivity, specificity, area under the curve (AUC) and 95% confidence intervals (95% CI) of each scoring system were calculated. Logistic regression analysis was used to analyse the related factors affecting the prognosis of these patients. A P value <0.05 on a two-sided test was considered statistically significant.
Results
One hundred fifty-three patients with COPD complicated by MODS were enrolled in the study (Figure 1), with a mean age of 77±10 years (range, 60-101 years). Ninety-five patients (62.1%) were males, and 89 patients (58.2%) required mechanical ventilation. Based on the 30-day outcome, these patients were divided into two groups: a 30-day survival group (59 cases) and a 30-day non-survival group (94 cases). The overall mortality rate was 61.4%.
Figure 1.
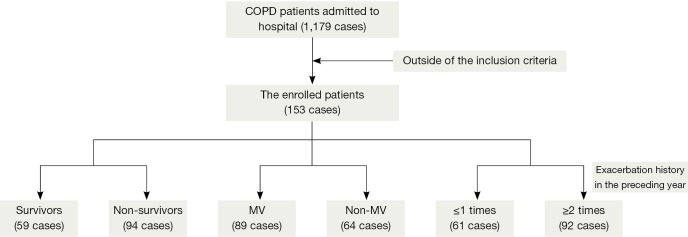
Flow chart of patient enrolment in this study. COPD, chronic obstructive pulmonary disease.
Table 1 lists the general characteristics of the patients’ conditions. The number of failing organs in the non-survival group was significantly higher than in the survival group (P<0.001). There were statistically significant differences in urine output, serum blood urea nitrogen (BUN), and serum creatinine between the survivors and non-survivors (P<0.05). All of the objective scoring models in survivors, including the APACHE II, APACHE III, MODS, SAPS II and SOFA scores, were significantly lower than those of the non-survivors (P<0.001). However, the length of the ICU stay in the survival group was longer than that of the non-survival group.
Table 1. Demographic and biochemical data of the enrolled patients.
| Characteristics | Outcomes of patients (n=153) |
P value | |
|---|---|---|---|
| Survivors (n=59) | Non-survivors (n=94) | ||
| Age (years) | 77±10 | 76±9 | 0.422 |
| Gender (No., %) | 0.367 | ||
| Male | 34 (22.2) | 61 (39.9) | |
| Female | 25 (16.3) | 33 (21.6) | |
| Number of failing organs* | 3 [2, 3] | 4 [3, 5] | <0.001 |
| Acute renal failure (%) | 5 (3.3) | 41 (26.8) | |
| Vital signs and laboratory tests | |||
| Body temperature (°C) | 37.3±1.3 | 37.7±1.8 | 0.067 |
| Heart rate (beats/min) | 105±31 | 113±31 | 0.131 |
| Mean arterial pressure (mmHg) | 82±26 | 79±22 | 0.401 |
| Urine output (mL/24 hours)* | 1,983±1,189 | 1,472±1,273 | 0.014 |
| WBC count (109/L) | 11.6±6.2 | 14.8±6.1 | 0.148 |
| Platelet count (109/L) | 151±69 | 117±75 | 0.069 |
| Serum albumin (g/L) | 30.5±6.8 | 30.8±9.5 | 0.824 |
| Serum glucose (mmol/L) | 9.5±4.5 | 10.1±4.8 | 0.464 |
| Serum BUN (mmol/L)* | 14.2±11.1 | 20.6±13.8 | 0.003 |
| Serum creatinine (µmol/L)* | 80.0 (60.3, 113.0) | 127.0 (89.0, 215.5) | <0.001 |
| Scoring systems | |||
| APACHE II* | 19±7 | 28±8 | <0.001 |
| APACHE III* | 71±29 | 101±29 | <0.001 |
| MODS* | 6±3 | 9±3 | <0.001 |
| SAPS II* | 44±16 | 62±17 | <0.001 |
| SOFA | 6±3 | 12±4 | <0.001 |
| Mechanical ventilation (No.) | 32 | 57 | 0.435 |
| Length of ICU stay (days)* | 17±11 | 13±7 | 0.013 |
data with a normal distribution are presented as the mean ± SD. Quantitative data with a non-normal distribution are presented as the mean (25th and 75th percentiles). Qualitative data are presented as n (%). *, represents the comparison between survivors and non-survivors, P<0.05. WBC counts, white blood cell counts; BUN, blood urea nitrogen; ICU, intensive care unit; APACHE, Acute Physiology and Chronic Health Evaluation; MODS, multiple organ dysfunction syndrome; SAPS, Simplified Acute Physiology Score II; SOFA, Sepsis-related Organ Failure Assessment; SD, standard deviation.
The power of each scoring system (APACHE II, APACHE III, MODS, SAPS II and SOFA) for predicting the outcome for the overall study population is shown in Table 2 and Figure 2. The sensitivity (0.936) and the AUC (0.875) of the SOFA score at a cut-off point of 6.5 was remarkably higher than those of the other scoring models, but the specificity of the SOFA score (0.695) was inferior to that of the APACHE II score (0.865), which was the most accurate model.
Table 2. Prognostic values of each scoring model for the enrolled patients.
| Scoring models | Cut-off | Se | Sp | Youden index | AUC | 95% CI |
|---|---|---|---|---|---|---|
| APACHE II | 25.5 | 0.617 | 0.865 | 0.481 | 0.789 | 0.714-0.863 |
| APACHE III | 86.0 | 0.649 | 0.797 | 0.446 | 0.775 | 0.699-0.851 |
| MODS | 6.5 | 0.777 | 0.678 | 0.455 | 0.739 | 0.656-0.823 |
| SAPS II | 53.5 | 0.681 | 0.814 | 0.494 | 0.802 | 0.729-0.875 |
| SOFA | 6.5 | 0.936 | 0.695 | 0.631 | 0.875 | 0.817-0.932 |
Se, sensitivity; Sp, specificity; ROC curve, receiver operating characteristic curve; APACHE, the acute Physiology and Chronic Health Evaluation; MODS, multiple organ dysfunction syndrome; SAPS, Simplified Acute Physiology Score II; SOFA, Sepsis-related Organ Failure Assessment; AUC, area under the curve; 95% CI, 95% confidence interval.
Figure 2.
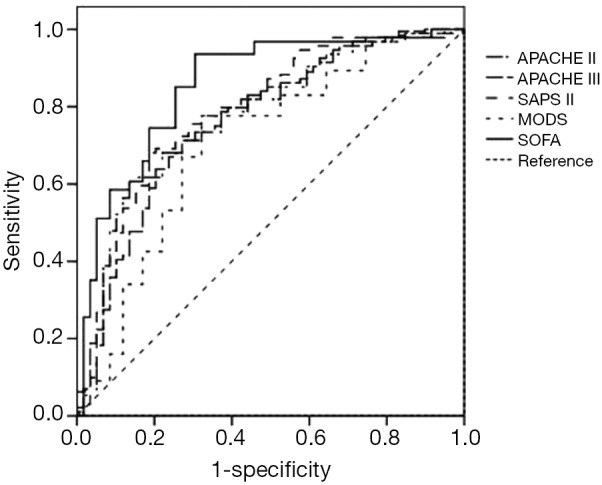
The ROC curves of the scoring systems for predicting the prognosis of enrolled patients (n=153). MODS, multiple organ dysfunction syndrome; SOFA, Sepsis-related Organ Failure Assessment; APACHE, the acute Physiology and Chronic Health Evaluation; SAPS, Simplified Acute Physiology Score II; ROC, receiver operating characteristic.
These five scoring models were also used to assess the prognosis of patients requiring mechanical ventilation, and the ROC curves of these assessments are depicted in Table 3 and Figure 3. The sensitivity (0.902) and the AUC (0.890) of the SOFA score at a cut-off point of 7.5 was remarkably higher than those of the other scoring models, but the specificity of the SOFA score (0.786) was inferior to that of the APACHE II score (0.893), which was the most accurate model. The combined prognostic value for patients of the two scoring models (APACHE II and SOFA) was not significantly improved (Table 3).
Table 3. Prognostic values of each scoring model for the enrolled patients requiring mechanical ventilation.
| Scoring models | Cut-off | Se | Sp | Youden index | AUC | 95% CI |
|---|---|---|---|---|---|---|
| APACHE II | 25.5 | 0.689 | 0.893 | 0.581 | 0.800 | 0.699-0.904 |
| APACHE III | 86.0 | 0.689 | 0.821 | 0.510 | 0.773 | 0.663-0.883 |
| MODS | 7.5 | 0.754 | 0.714 | 0.468 | 0.759 | 0.643-0.876 |
| SAPS II | 53.0 | 0.770 | 0.821 | 0.592 | 0.813 | 0.704-0.923 |
| SOFA | 7.5 | 0.902 | 0.786 | 0.687 | 0.890 | 0.813-0.967 |
| Combined diagnosis | 0.548 | 0.934 | 0.786 | 0.720 | 0.890 | 0.803-0.967 |
Se, sensitivity; Sp, specificity; ROC curve, receiver operating characteristic curve; APACHE, the acute Physiology and Chronic Health Evaluation; MODS, multiple organ dysfunction syndrome; SAPS, Simplified Acute Physiology Score II; SOFA, Sepsis-related Organ Failure Assessment; AUC, area under the curve; 95% CI, 95% confidence interval.
Figure 3.
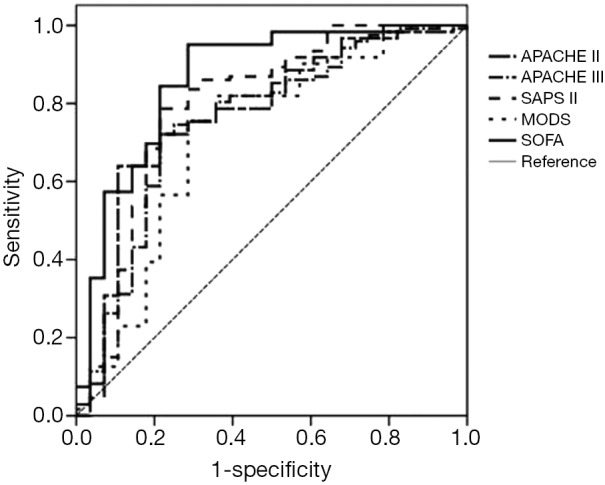
The ROC curves of the scoring systems for predicting the prognosis of enrolled patients requiring mechanical ventilation (n=89). ROC, receiver operating characteristic; SOFA, Sepsis-related Organ Failure Assessment.
To determine the factors related to the prognosis, logistic regression analysis was performed. Four indicators were associated with the prognosis of elderly patients with MODS associated with the AE of COPD: the exacerbation history, SOFA scores, number of failing organs, and length of the ICU stay, and the odds ratio (OR) values were 5.910, 1.387, 3.617 and 0.927, respectively (Table 4).
Table 4. Factors related to the prognosis of patients with MODS associated with acute COPD exacerbation using logistic regression analysis.
| Variable | B | Se | Wald | df | P value | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| Exacerbation history in the preceding year | 1.777 | 0.354 | 25.163 | 1 | 0.000 | 5.910 | 2.952-11.832 |
| SOFA | 0.327 | 0.068 | 23.165 | 1 | 0.000 | 1.387 | 1.214-1.585 |
| Number of failing organs | 1.339 | 0.315 | 18.041 | 1 | 0.000 | 3.617 | 2.057-7.077 |
| Length of ICU stay | –0.076 | 0.035 | 4.756 | 1 | 0.029 | 0.927 | 0.865-0.992 |
MODS, multiple organ dysfunction syndrome; COPD, chronic obstructive pulmonary disease; SOFA, Sepsis-related Organ Failure Assessment; ICU, intensive care unit; Se, sensitivity; Sp, specificity.
Table 4 shows that the exacerbation history is a high-risk factor for a poor prognosis of COPD. A ROC curve was employed to assess the prognosis of the patients studied in relation to their exacerbation history. The results showed that the AUC of the exacerbation history was 0.892, with a sensitivity of 0.851 and a specificity of 0.797 at a cut-off point of 2 (Figure 4). SOFA scores were increased in parallel with the fit of the frequency of exacerbation, with significant differences between two groups (≤1 times group and ≥2 times group) (P<0.001). A more frequent exacerbation history corresponded to higher scores and a worse prognosis (Figure 5).
Figure 4.
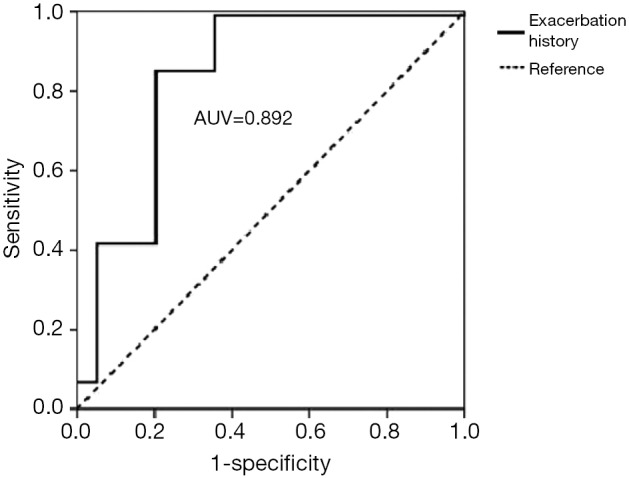
The ROC curve of the SOFA score for predicting the outcome of enrolled patients based on exacerbation frequency. ROC, receiver operating characteristic; SOFA, Sepsis-related Organ Failure Assessment.
Figure 5.
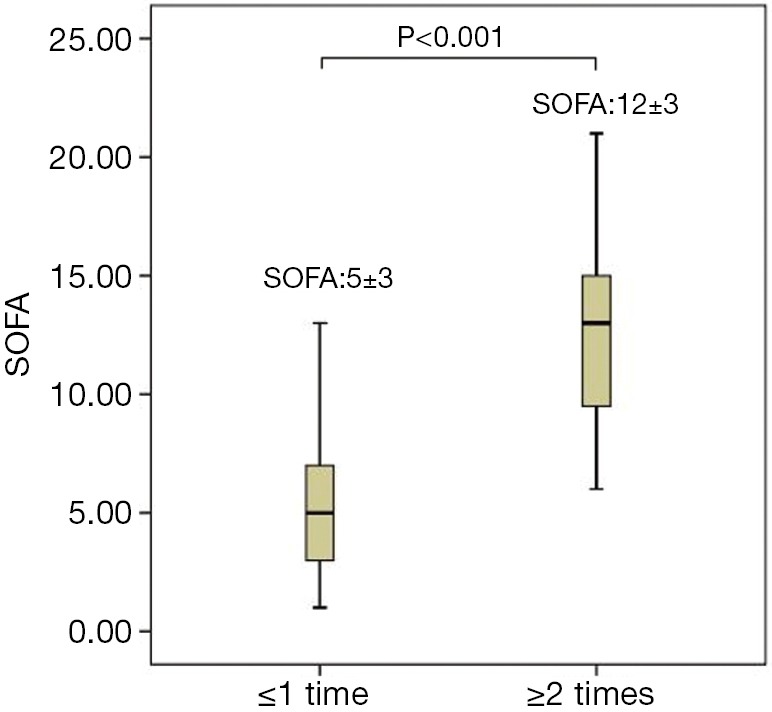
The difference in SOFA scores between different levels of exacerbation frequency in the preceding year before admission to the hospital in patients with COPD. SOFA, Sepsis-related Organ Failure Assessment; COPD, chronic obstructive pulmonary disease.
Discussion
In this retrospective study, one third of the patients were women suffering from COPD, and the proportion is gradually increasing. Some published studies have indicated that smoking increases the risk of COPD (18,19), and passive smoke exposure is also a risk factor (20-22), particularly for Chinese rural women. Cooking mainly depends on biomass fuels, including straw and firewood in rural areas, and the exhaust gases, which contain sulphur dioxide and other harmful substances, are likely to increase the pathogenesis of COPD (23).
Compared with the survival group, the number of failing organs in our study was remarkably higher in the non-survival group. The results of logistic regression analysis showed that the number of failing organs was a risk factor for a poor prognosis of elderly COPD patients with MODS. A previous study indicated that a higher number of failing organs was associated with a higher prognostic assessment score and corresponded to a higher mortality rate in 197 patients with MODS (24). The length of the ICU stay in non-survivors was less than that of survivors and was an advantageous factor for predicting the prognosis in the present study. The above results indicated that longer positive treatment corresponded to a better prognosis for this critical illness.
Age was not an independent factor for prognosis and showed no significant difference between survivors and non-survivors. However, age is a component of APACHE scores, and it was reported that age was a prognostic factor in the supporting study (25). Study of larger sample sizes may be needed to reconcile our results.
The scoring models showed a better performance compared to the severity of illness systems. The present study is most likely the first report to determine that the SOFA and SAPS II scores show very good discrimination (AUC >0.80) for predicting the prognosis of elderly patients suffering from MODS associated with COPD compared with APACHE II, APACHE III and MODS scores. Additionally, the SOFA scoring system may be more appropriate to use in predicting outcomes due to its high sensitivity, excellent predictive value, simplicity and ease of assessment. The SOFA was the only one of the five scoring models to be retained in the multivariable analysis as an independent risk factor for 30-day mortality, and some previous studies had demonstrated that the SOFA score reliably predicted long-term outcomes in patients with trauma (26) or acute myocardial infarction (AMI) (27). Consequently, the SOFA score could be a good predictor for short-term as well as long-term outcomes.
The SOFA score also increased in parallel with the fit of exacerbation frequency, which was associated with mortality. The exacerbation history was an outstanding risk factor for a poor 30-day prognosis of patients with MODS caused by COPD in our study. Published studies verify that the frequency of COPD exacerbation in the preceding year is risk factor for mortality (28,29). In our study, we also assessed the prognosis of COPD through the number of previous acute onsets of COPD, and then drew the conclusion that the exacerbation history performs well in predicting the outcome of severe COPD patients (AUC: 0.892), with a sensitivity of 0.851 and a specificity of 0.797 at a cut-off point of 2, using the ROC curve.
Our study has two major limitations. First, this is a retrospective study, and some useful indicators were missed, such as the 6-min walk test, the COPD Assessment Test (CAT) and the Modified British Medical Research Council (mMRC) questionnaire, which also can predict outcomes of COPD patients. In addition, the data collected from one centre can lead to results that are not generalisable. However, these research results are important because they can guide the design of a prospective and observational study which could validate the conclusions of this study, and can allow the clinical significance of these findings to be further explored.
Conclusions
In summary, this study showed that SOFA scores determined at the onset of MODS in elderly patients with COPD were a reliable predictor of the prognosis. The exacerbation history, number of failing organs, and the SOFA score were risk factors of a poor prognosis, and the exacerbation history could also make an effective prediction of the outcome of COPD. It would be useful to combine the assessment scores with the other elements (number of exacerbations, etc.) to develop a new prediction model that would be more accurate, specifically for COPD. We suggest that a multi-centre prospective study with a larger sample size would be useful to evaluate the efficacy of these scoring models in predicting outcomes for elderly patients with MODS associated with AECOPD.
Acknowledgements
Written consent for publication was obtained from the patients or their legal family members. We express our gratitude to the nurses and doctors of the EICU and RICU of the Chinese PLA General Hospital for their help with this study. This work was supported by Projects fostering Capital Public Health by the Science and Technology Commission of Beijing.
Authors’ contributions: All authors are fully responsible for the presented analysis and conclusions drawn in the manuscript. Dr. Xiao: conceived the idea, designed and initiated the study, performed statistical analysis, drafted the manuscript, and approved the final version; Dr. Guo: designed and initiated the study, collected the data, and approved the final version; Dr. Su and Dr. Li: conceived the idea, designed and initiated the study, and approved the final version; Dr. Xie: conceived the idea, designed and initiated the study, amended and commented on the manuscript, and approved the final version.
Disclosure: The authors declare no conflict of interest.
References
- 1.Barnett M.An overview of assessment and management in COPD. Br J Community Nurs 2009;14:195-201. [DOI] [PubMed] [Google Scholar]
- 2.Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004;364:613-20. [DOI] [PubMed] [Google Scholar]
- 3.Burney P, Jithoo A, Kato B, et al. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty--a BOLD analysis. Thorax 2014;69:465-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimada H, Moriwaki Y, Kurosawa H, et al. Inflammatory mediator and organ dysfunction syndrome. Nihon Geka Gakkai Zasshi 1998;99:490-6. [PubMed] [Google Scholar]
- 5.Fang X, Wang X, Bai C.COPD in China: the burden and importance of proper management. Chest 2011;139:920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mapel DW, Dutro MP, Marton JP, et al. Identifying and characterizing COPD patients in US managed care. A retrospective, cross-sectional analysis of administrative claims data. BMC Health Serv Res 2011;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397-412. [DOI] [PubMed] [Google Scholar]
- 8.Hites M, Dell’Anna AM, Scolletta S, et al. The challenges of multiple organ dysfunction syndrome and extra-corporeal circuits for drug delivery in critically ill patients. Adv Drug Deliv Rev 2014;77:12-21. [DOI] [PubMed] [Google Scholar]
- 9.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [PubMed] [Google Scholar]
- 10.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 1991;100:1619-36. [DOI] [PubMed] [Google Scholar]
- 11.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995;23:1638-52. [DOI] [PubMed] [Google Scholar]
- 12.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957-63. [DOI] [PubMed] [Google Scholar]
- 13.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [DOI] [PubMed] [Google Scholar]
- 14.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [DOI] [PubMed] [Google Scholar]
- 15.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644-55. [DOI] [PubMed] [Google Scholar]
- 16.Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl 2003;41:46s-53s. [DOI] [PubMed] [Google Scholar]
- 17.Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J 2007;29:1224-38. [DOI] [PubMed] [Google Scholar]
- 18.Eisner MD, Balmes J, Katz PP, et al. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health 2005;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang JW, Chung S, Sundar IK, et al. Cigarette smoke-induced autophagy is regulated by SIRT1-PARP-1-dependent mechanism: implication in pathogenesis of COPD. Arch Biochem Biophys 2010;500:203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coultas DB. Health effects of passive smoking. 8. Passive smoking and risk of adult asthma and COPD: an update. Thorax 1998;53:381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin P, Jiang CQ, Cheng KK, et al. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. Lancet 2007;370:751-7. [DOI] [PubMed] [Google Scholar]
- 22.Hagstad S, Bjerg A, Ekerljung L, et al. Passive smoking exposure is associated with increased risk of COPD in never smokers. Chest 2014;145:1298-304. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Zhou Y, Wang X, et al. Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South China. Thorax 2007;62:889-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao K, Su L, Han B, et al. Prognosis and weaning of elderly multiple organ dysfunction syndrome patients with invasive mechanical ventilation. Chin Med J (Engl) 2014;127:11-7. [PubMed] [Google Scholar]
- 25.Cooper GS, Bellamy P, Dawson NV, et al. A prognostic model for patients with end-stage liver disease. Gastroenterology 1997;113:1278-88. [DOI] [PubMed] [Google Scholar]
- 26.Ulvik A, Kvåle R, Wentzel-Larsen T, et al. Multiple organ failure after trauma affects even long-term survival and functional status. Crit Care 2007;11:R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang SS, Chen YH, Lu TM, et al. Application of the Sequential Organ Failure Assessment score for predicting mortality in patients with acute myocardial infarction. Resuscitation 2012;83:591-5. [DOI] [PubMed] [Google Scholar]
- 28.Seneff MG, Wagner DP, Wagner RP, et al. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA 1995;274:1852-7. [PubMed] [Google Scholar]
- 29.Aburto M, Esteban C, Moraza FJ, et al. COPD exacerbation: mortality prognosis factors in a respiratory care unit. Arch Bronconeumol 2011;47:79-84. [DOI] [PubMed] [Google Scholar]


