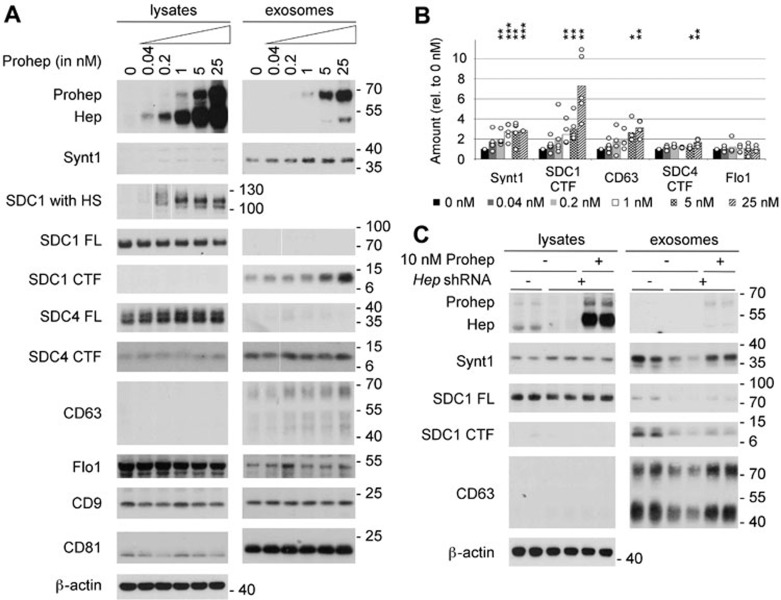
Figure 1.
Heparanase stimulates the production of syntenin-1-containing exosomes. (A) Exosome production was evaluated after overnight conditioning of MCF-7 cells with increasing concentrations of proheparanase (0.04-25 nM) and compared to that of cells not receiving proheparanase (0 nM). Exosomes were collected from equivalent amounts of culture medium, conditioned by equal numbers of cells, for equal lengths of time. For each condition both the lysate and exosomal fractions were analyzed by western blot, using cognate antibodies against heparanase, monitoring the conversion of proheparanase (Prohep) into mature heparanase (Hep) and against different exosomal markers: syntenin-1 (Synt1), syndecan-1 (SDC1), syndecan-4 (SDC4), CD63, flotillin-1 (Flo1), CD9 and CD81. Syndecan-1, which is a hybrid heparan sulfate (HS)/chondroitin sulfate proteoglycan, was analyzed using two different approaches. In one approach, the samples were digested with both heparitinase and chondroitinase ABC, removing all glycosaminoglycan chains and enabling visualization of the full-length syndecan core proteins (SDC1 FL) as sharp bands. In the other approach, the samples were digested with chondroitinase ABC only, leaving the HS on the syndecans (SDC1 with HS); comparison of 'SDC1 with HS' and 'SDC1 FL' yields information on the mass of HS on syndecans. Because of the heterogeneity in HS chain length, syndecan-1 with HS is smeared over a wide mass range in the absence of heparanase activity (and is therefore hardly visible in western blot, as illustrated by lane 1 of the lysates). With increasing heparanase activity, the HS chains on syndecan-1 are trimmed to shorter chains of more or less the same length, syndecan-1 with HS migrating as one or a few bands that are readily visualized in western blot (as illustrated by lane 6 of the lysates). Note that cell lysates contain mainly full-length syndecan core proteins; the opposite is true for exosomes, where hardly any full-length syndecan is detected and C-terminal fragments (CTFs) represent the dominant form. β-actin was used as a loading control for the lysates. Western blots are representative of five independent experiments. (B) Histogram representing the quantification of the exosomal levels of syntenin-1 (Synt1), syndecan-1 CTF (SDC1 CTF), CD63, syndecan-4 CTF (SDC4 CTF) and flotillin-1 (Flo1) in response to the addition of increasing concentrations (0 nM till 25 nM) of proheparanase. Values are relative to the exosomal levels measured in absence of exogenously added proheparanase. Bar heights represent mean values, calculated from five independent experiments. Individual data points are shown as white dots on top of the corresponding bars. *P < 0.1, **P < 0.05, ***P < 0.01 (Student's t-test, assuming normal distribution of the data points). (C) Knockdown of endogenous heparanase reduces the production of syntenin-1-containing exosomes, which can be rescued by the addition of exogenous proheparanase. Duplicate lanes show the results of two independent experiments, run side by side. B16-F10 cells are sham-transfected (−) or stably transfected with a shRNA targeting murine heparanase (+). To rescue the effects of endogenous heparanase knockdown, 10 nM human proheparanase was added to the cells. Heparanase, syntenin-1, syndecan-1 full-length (SDC1 FL), syndecan 1 CTF (SDC1 CTF) and CD63 were analyzed by western blot. Positions of molecular weight markers (in kDa) are indicated on the right of each blot. Note that (because of differences in glycosylation) the Mr of human heparanase is slightly larger than that of mouse heparanase.