Fig. 1.
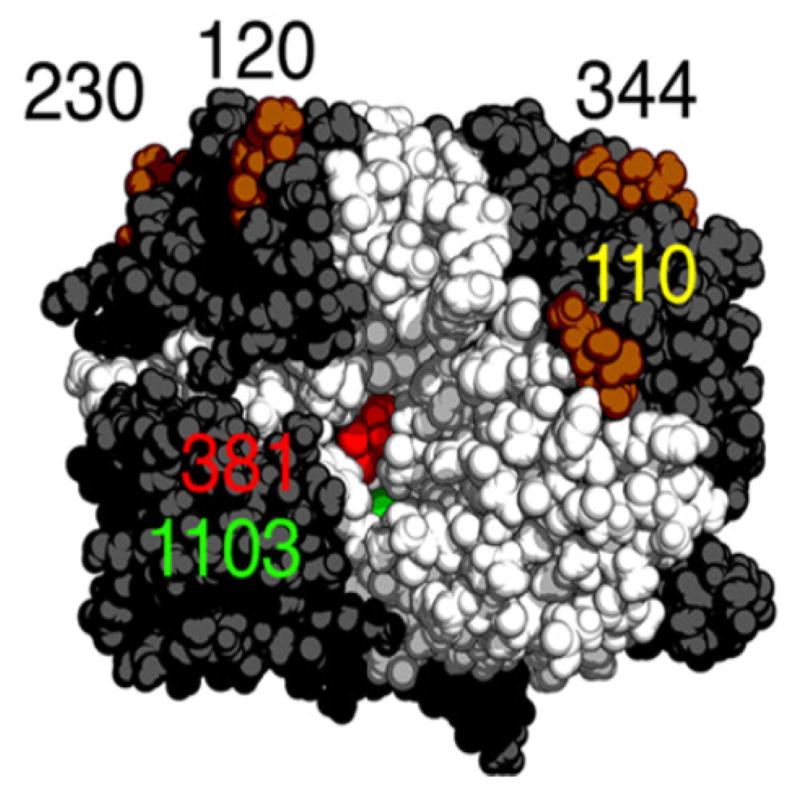
The van der Waals surface of human GGT with the active site cleft facing the viewer. The large subunit (dark gray) and the small subunit (white) are shown with the catalytic Thr-381 (red) in the deepest part of the small subunit cleft. Four of the seven potential glycosylation sites are seen in this orientation, asparagine 110, 120, 230 and 344, and the basal N-acetyl glucosamine residue, which was identified in the crystal structure at each of these sites, is represented as dark orange van der Waals spheres. An anion-binding site (green) within the small subunit cleft is labeled 1103. This figure was originally published in The Journal of Biological Chemistry. West, M.B., Chen, Y., Wickham, S., Heroux, A., Cahill, K., Hanigan, M.H., Mooers, B.H.M., Novel insights into eukaryotic gamma-glutamyl transpeptidase 1 from the crystal structure of the glutamate bound human enzyme. J. Biol. Chem. 2013; 288(44):31902–31913. © the American Society for Biochemistry and Molecular Biology
