Fig. 4.
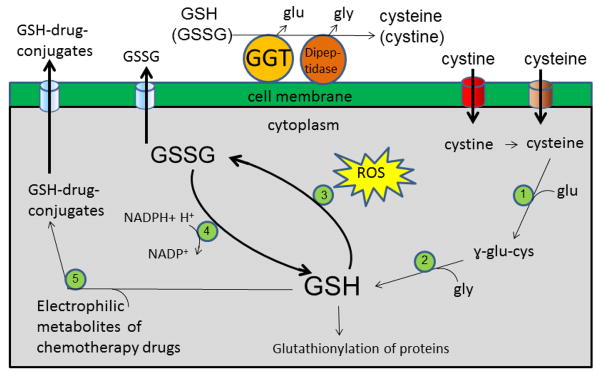
Gamma-glutamyl transpeptidase (GGT)-positive tumors cleave extracellular reduced and oxidized glutathione (GSH and GSSG) providing an additional source of cysteine for intracellular GSH synthesis. GGT cleaves glutamate from GSH and GSSG. The cysteinylglycine dipeptides can be cleaved by any of several dipeptidases that are present on the surface of the cell. The glutamate, glycine, cystine and cysteine that are released from GSH and GSSG, are transported into the cell by the standard amino acid transporters. Cystine is taken up by the xc− cystine/glutamate antiporter (red). Cysteine is taken up by the ACS transporter (brown). Once inside the cell, cystine is reduced to cysteine by the strongly reducing environment of the cytoplasm. The first step in GSH synthesis is catalyzed by gamma-glutamyl cysteine synthetase (1) and the second step is catalyzed by GSH synthetase (2). Tumors are under redox stress which can be further increased by pro-oxidant therapy. GSH levels are depleted in tumors by several pathways. GSH is oxidized to GSSG as part of an ROS detoxification system that is present in both the cytoplasm and mitochondria. GSH peroxidases (3) catalyze the oxidation of GSH to GSSG. GSSG and be reduced to GSH by GSH reductase (4) and NADPH. However, under extreme oxidative stress GSSG is transported out of the cell by the multidrug resistance protein (MRP) transporters (blue). GSH is also depleted from the cell by binding to the electrophilic metabolites of chemotherapy drugs, which is catalyzed by GSH S-transferases (5). The GSH-conjugates are transported out of the cell by the MRP transporters (blue). Intracellular GSH can also be depleted by binding to intercellular proteins, a process known as protein glutathionylation.
