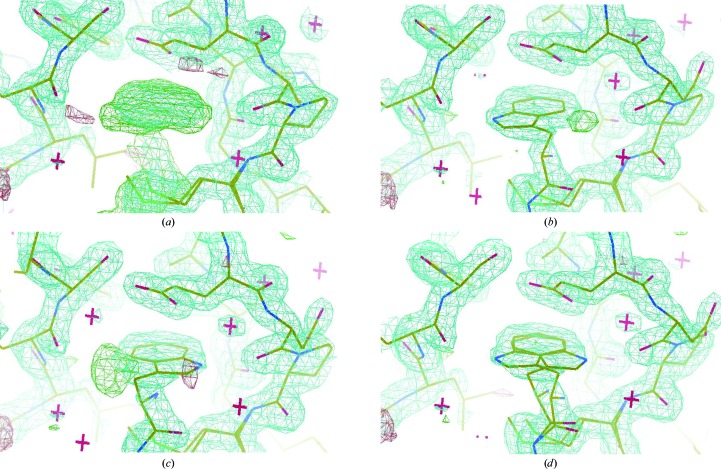
Figure 3.
Trp2 double-conformation assignment during refinement. (a) OMIT maps of the crucial adhesion arm provided a clear indication of the closed conformation of the protein in the crystal, with the Trp2 residue fitting into the protein’s own acceptor pocket. (b, c, d) The electron-density map corresponding to the Trp2 side chain was found to correspond to a double conformation of the indole moiety (50% occupancy each). Strong residual electron density was in fact evident when only one of the two conformations was refined in the model. The two flipped conformations are essentially coplanar, with the six-membered ring almost perfectly coinciding in the two orientations. The maps are contoured at the 1.0 and 1.5σ levels.