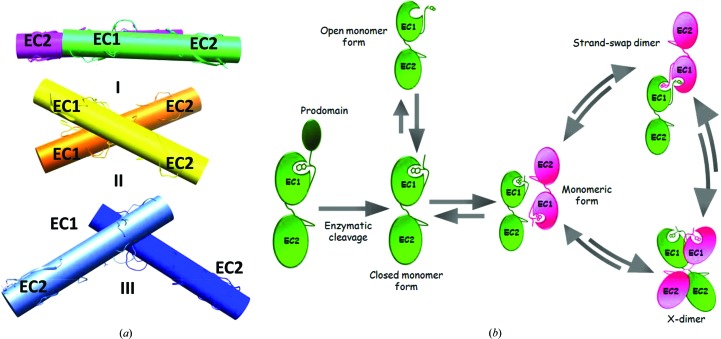
Figure 4.
(a) The relative orientation of next-neighbour molecules in different cadherin forms and packing arrangements. (I) The packing arrangement of the closed-conformation human P-cadherin EC1-EC2 monomer. (II) The closed-conformation mouse E-cadherin EC1-EC2 X-dimer (PDB entry 1ff5). (III) The human E-cadherin EC1-EC2 strand-swap dimer (PDB entry 2o72). (b) Schematic representation of a putative model of a multistep cadherin dimerization pathway. Upon enzymatic cleavage, the cadherin prodomain is removed to afford a mature cadherin protein primed for adhesion. In the early stages of the dimerization process, an equilibrium between open and closed monomers exists. Cellular polarization events lead to an increase in the protein concentration, ultimately triggering a concerted and highly dynamic cadherin-recognition process. Transient, metastable interactions may allow molecules from opposing cells to orient themselves in an antiparallel fashion, as shown by the P-cadherin EC1-EC2 structure reported here, and to subsequently slide into a dimeric/adhesive form. The dimerization process can then dynamically revert to again provide monomeric cadherin as a result of mechanical forces. The lengths of the arrows in this scheme are purely indicative and do not reflect the relative likelihoods of the different pathways.