Abstract
Objectives
Yersiniosis, a foodborne infection of zoonotic origin caused by the bacteria Yersinia enterocolitica and Yersinia pseudotuberculosis, is a reportable disease in 38 states. Both sporadic and foodborne outbreaks of yersiniosis have been reported in the U.S., with annual occurrence of an estimated 98,000 episodes of illness, 533 hospitalizations, and 29 deaths. We analyzed surveillance data from nine non-FoodNet-participating U.S. states during the period 2005–2011 to describe the epidemiology of this disease.
Methods
As part of a passive surveillance system, laboratory-confirmed cases of yersiniosis were reported to state health departments in Arizona, Illinois, Michigan, Missouri, Nebraska, North Carolina, South Carolina, Washington, and Wisconsin. We calculated overall, age-, and race-specific annual incidence rates per 100,000 population using 2010 Census data as the denominator. We used Poisson regression to examine seasonal variation and annual incidence trends by race, age group, and overall.
Results
The average annual incidence of yersiniosis was 0.16 cases per 100,000 population during 2005–2011. We observed a statistically significant decreasing annual trend of yersiniosis incidence among African Americans <5 years of age (p<0.01), whereas white people aged 19–64 years (p=0.08) and Hispanic people (p=0.05) had an overall increasing annual incidence of yersiniosis. We observed higher incidence during October–December (p<0.01) and January–March (p=0.03) quarters among African Americans, whereas white people had a higher incidence during April–June (p=0.05).
Conclusion
This multistate analysis revealed differences in the epidemiology of yersiniosis by race/ethnicity that may be useful for future research and prevention efforts. While this study was consistent with the FoodNet report in recognizing the high and declining incidence among African American children and winter seasonality among African Americans, our study also identified April–June seasonality among the white population.
An estimated 98,000 episodes of yersiniosis, including 533 hospitalizations and 29 deaths, occur annually in the United States.1 Yersiniosis is a foodborne infection of zoonotic origin caused by the bacteria Yersinia enterocolitica and Yersinia pseudotuberculosis.2 Yersiniosis often presents as an invasive diarrheal disease characterized by fever, abdominal pain, mucous- and blood-containing stools with fecal leukocytes, and positive stool cultures.3–6 Mesenteric lymphadenitis mimicking appendicitis especially in older children and adults has also been noted.4,7,8 Yersiniosis has a propensity for extraintestinal spread, especially among immunocompromised hosts and people with iron overload.9
Consumption of chitterlings (pig intestines), a traditional dish served at Thanksgiving and Christmas, and other pork products, as well as exposure to animals and consumption of unpasteurized milk have been identified as risk factors during outbreak investigations.10–12 Several outbreaks of yersiniosis were linked to pasteurized milk as well.13,14 However, most yersiniosis cases are sporadic and risk factors have not been studied extensively.3
Yersiniosis is reportable in 38 states. However, there are few published analyses available from states that have only passive surveillance for yersiniosis. In 2012, FoodNet, an active surveillance network for foodborne infections conducted by the Centers for Disease Control and Prevention (CDC) and partners in 10 U.S. states, reported a 66% reduction in the incidence of yersiniosis in children <5 years of age during 1996–2009. The reduction has been attributed to educational efforts, primarily in Georgia.15
One of the uses of surveillance data is hypothesis generation, which may inform future research. Descriptive epidemiologic analyses may also identify population subgroups with a relatively higher incidence of disease, thus directing resource prioritization for appropriate prevention interventions. To help direct future study and prevention of yersiniosis and to compare the results of analysis of passive surveillance with CDC's active system (FoodNet), we analyzed data from nine U.S. states that are not participants in FoodNet to describe the epidemiology of yersiniosis during 2005 through 2011, a time period in which data were available from all participating states.
METHODS
Surveillance data from a convenience sample of nine states—Arizona, Illinois, Michigan, Missouri, Nebraska, North Carolina, South Carolina, Washington, and Wisconsin—reported from 2005 through 2011, were pooled for analysis of variables for which data were available in all states. Laboratory-confirmed cases of yersiniosis with identification of the organism in stool or blood culture were reported to state health departments by health-care providers, laboratories, and local health departments. Reports are required to be submitted using either the electronic disease surveillance system or the communicable disease report form to the local health departments within 24 hours in Michigan and Washington; 72 hours in Missouri, South Carolina, and Wisconsin; five business days in Arizona; and seven days in Illinois and Nebraska. The reports generally include age, sex, race/ethnicity, date of illness onset, and date of diagnosis. In North Carolina, yersiniosis is reportable (within 24 hours) under the condition “Foodborne Disease, Other”; therefore, it is not listed separately by its name on North Carolina's list of reportable diseases.
We calculated frequency of cases by age categories (<1 year, 1–4 years, 5–18 years, 19–64 years, and >64 years), sex, race (African American, Asian, white, and other), and ethnicity (Hispanic or Latino, non-Hispanic or Latino). We used age categories that are comparable with the most recently published FoodNet report.15 We calculated overall annual incidence of reported confirmed yersiniosis cases per 100,000 population for each of these states using 2010 U.S. Census data.16 We also calculated incidence by age group, race, and ethnicity. We combined the lowest two age categories while calculating incidence, as the denominator data were available for those <5 years of age but not for those <1 year of age. We calculated relative risks (RRs) and 95% confidence intervals (CIs) to compare the average annual incidence among different races by age categories. We used chi-square tests or Fisher's exact tests to determine statistically significant differences in frequencies based on these demographic characteristics. We examined annual trends of overall incidence of yersiniosis as well as incidence by race, ethnicity, and age using SAS® GENMOD.17 We modeled the raw incidence data through the use of Poisson regression with a logarithmic link function and a log (population) offset term, and tested the presence of a crude calendar year effect. To check for variations in yersiniosis incidence during different times of the year by race and ethnicity, we categorized months of onset into four quarters: January–March, April–June, July–September, and October–December. We included this categorical variable in the aforementioned Poisson regression model and tested for seasonal increases in incidence compared with the reference category (July–September).
Because Illinois and Washington had data on chitterlings exposure, we performed a sub-analysis of these cases to compare the odds of exposure to chitterlings among races by age category, among children <5 and ≥5 years of age. Washington also had data on raw pork exposure and unpasteurized milk consumption. We compared the odds of exposure to these behaviors among different races. We calculated exact mid-p confidence limits around the odds ratios (ORs). Exposure data were not available for other states. We considered statistical test results significant at a=0.05. All analyses were performed using Microsoft® Excel® and SAS® 9.3.18
RESULTS
During 2005–2011, 721 confirmed yersiniosis cases were reported among the nine states (Table 1). Three hundred ninety-one (54%) cases were female (range: 49% in Arizona to 61% in Nebraska). There was no statistically significant difference in sex distribution of yersiniosis cases in different states (p=0.73) (data not shown). Among the respondents, 342 (47%) were white, 146 (20%) were African American, 30 (4%) were Asian, 18 (2%) were other races, and 185 (26%) had race recorded as unknown or missing. Fifty (7%) were Hispanic or Latino, 402 (56%) were non-Hispanic or non-Latino, and 269 (37%) had ethnicity recorded as unknown or missing (Table 1).
Table 1.
Demographic characteristics of yersiniosis cases in nine U.S. states,a 2005–2011 (n=721)
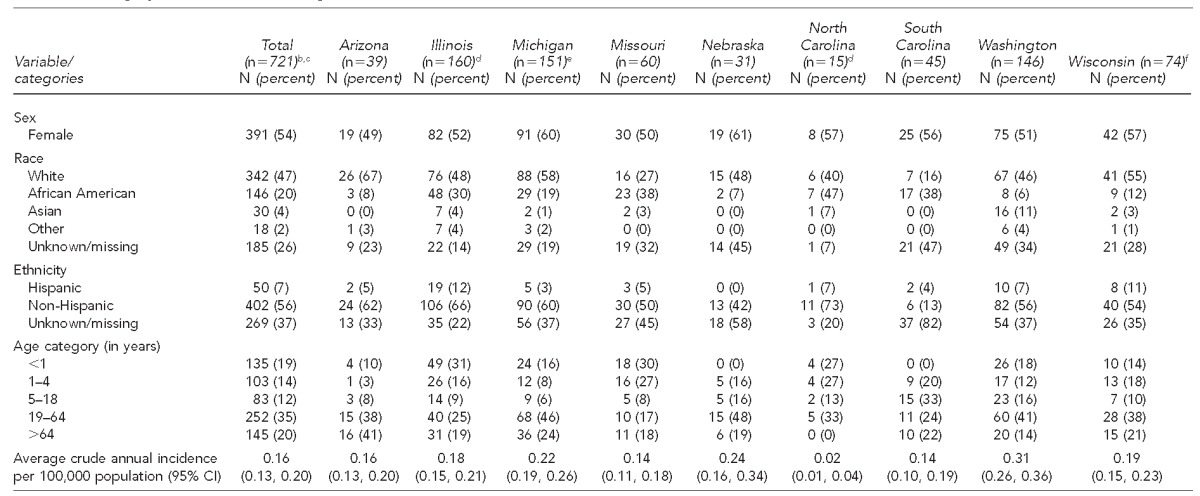
The nine states were Arizona, Illinois, Michigan, Missouri, Nebraska, North Carolina, South Carolina, Washington, and Wisconsin.
bGender information was missing in two cases.
cAge information was missing in three cases.
dGender information was missing in one case.
eAge information was missing in two cases.
fAge information was missing in one case.
CI = confidence interval
Figure 1. Annual incidence of yersiniosis per 100,000 population, by age group, among African Americans: nine U.S. states,a 2005–2011
aThe nine states were Arizona, Illinois, Michigan, Missouri, Nebraska, North Carolina, South Carolina, Washington, and Wisconsin.
The overall annual incidence in these nine states was 0.16 per 100,000 population. Annual reported incidence was highest in Washington (0.31), followed by Nebraska (0.24), Michigan (0.22), Wisconsin (0.19), Illinois (0.18), Arizona (0.16), South Carolina (0.14), Missouri (0.14), and North Carolina (0.02) (Table 1). Although the annual incidence was highest in 2006 (0.19), it was lowest in 2005 and 2010 (0.14) (data not shown). We did not observe a statistically significant linear decrease in the overall population incidence (p=0.59). While we observed a statistically significant decreasing trend in incidence among the African American population, from 0.28 in 2005 to 0.16 in 2011 (p<0.05), no significant trend in annual incidence over time was observed among white people (0.07 in 2005 and 0.11 in 2011, p=0.19) and Asians (0.10 in 2005 and 0.15 in 2011, p=0.99). Among Hispanic people, the average annual incidence was 0.10 and there was a statistically significant increasing trend, from 0.04 in 2005 to 0.22 in 2010 and 0.10 in 2011 (p=0.05) (data not shown).
We observed a 56% decrease in the incidence of yersiniosis in African American children <5 years of age (from 2.93 per 100,000 population in 2005 to 1.30 per 100,000 population in 2011, p=0.01) (Figure 1). The largest decline was in Illinois (from 6.0 per 100,000 population in 2005 and 7.5 per 100,000 population in 2006 to 2.2 per 100,000 population in 2011) and in Michigan (from 4.0 per 100,000 population in 2005 and 6.0 per 100,000 population in 2007 to 1.0 per 100,000 population in 2011) (data not shown). No significant trend was observed among African Americans in other age groups (Figure 1). Among white children <5 years of age, we observed a gradual increase in incidence from 2005 (0.33 per 100,000 population) through 2009 (0.48 per 100,000 population), followed by a decrease in 2011 (0.29 per 100,000 population). However, no significant linear trend was observed in this group (p=0.76). Among white adults aged 19–64 years, we observed an increasing trend in incidence, from 0.03 per 100,000 population in 2005 to 0.09 per 100,000 population in 2011 (p=0.08), but no statistically significant trend was observed in other race-age groups (data not shown).
Figure 1.
Annual incidence of yersiniosis per 100,000 population, by age group, among African Americans: nine U.S. states,a 2005–2011
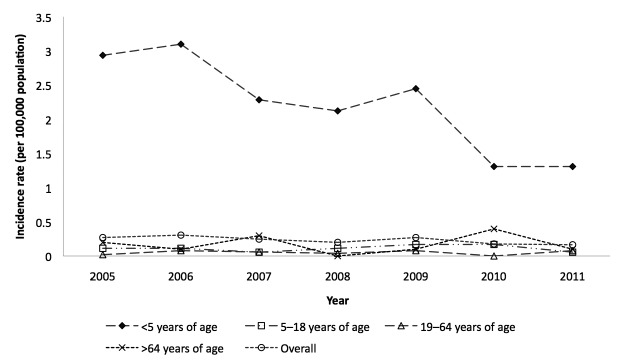
aThe nine states were Arizona, Illinois, Michigan, Missouri, Nebraska, North Carolina, South Carolina, Washington, and Wisconsin.
Among children aged <5 years, Asians and African Americans had 2.11 (95% CI 1.45, 3.06) and 2.45 (95% CI 2.02, 2.97) (both p<0.01) times higher average annual incidence compared with white people. Similarly, among 5- to 18-year-olds, Asians and African Americans had 3.57 (95% CI 1.51, 8.46, p<0.01) and 1.92 (95% CI 1.07, 3.46, p=0.03) times higher average annual incidence of yersiniosis compared with white people. There was no statistically significant difference in average incidence by race among adults aged 19–64 years and among adults >64 years of age (Table 2).
Table 2.
Average annual incidence per 100,000 population and relative risk of yersiniosis, by race and age group: nine U.S. states,a 2005–2011 (n=518)
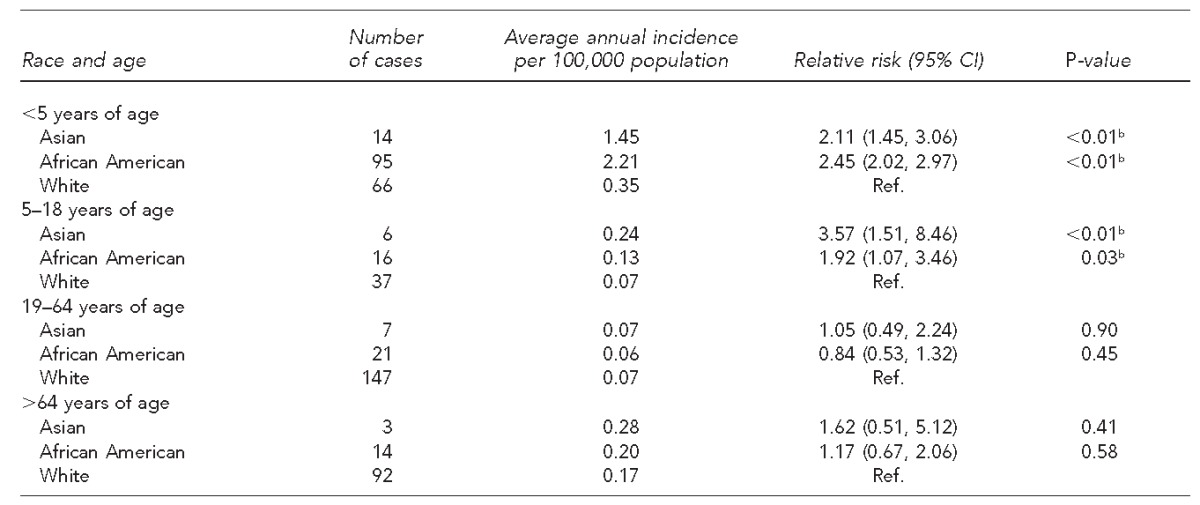
The nine states were Arizona, Illinois, Michigan, Missouri, Nebraska, North Carolina, South Carolina, Washington, and Wisconsin.
bStatistically significant at p=0.05
CI = confidence interval
Ref. = reference group
Figure 2. Number of cases of yersiniosis, by month and race/ethnicity: nine U.S. states,a 2005–2011
aThe nine states were Arizona, Illinois, Michigan, Missouri, Nebraska, North Carolina, South Carolina, Washington, and Wisconsin.
We observed a statistically significant seasonal variation among African Americans and white people. Among African Americans, the average number of cases reported during October–December (p<0.01) and January–March (p=0.03) was statistically significantly higher than during July–September. Among white people, the number of cases reported during the April–June quarter was higher than during the reference quarter (p=0.05). On the other hand, there was no statistically significant seasonal trend observed among Hispanic people (Figure 2).
Figure 2.
Number of cases of yersiniosis, by month and race/ethnicity: nine U.S. states,a 2005–2011
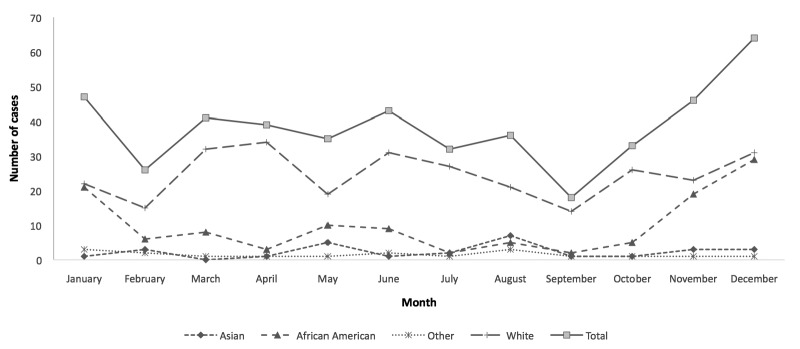
aThe nine states were Arizona, Illinois, Michigan, Missouri, Nebraska, North Carolina, South Carolina, Washington, and Wisconsin.
In the sub-analysis of cases reported in Illinois and Washington combined, African American children <5 years of age had 22.3 times higher odds of being exposed to chitterlings than their white counterparts (46% vs. 4%, OR=22.3, 95% CI 3.0, 246.0). On the other hand, among children aged ≥5 years, Asians had higher odds of being exposed to chitterlings than their white counterparts (25% vs. 3%, OR=13.0, 95% CI 1.7, 88.0). No statistically significant racial variation in exposure to raw pork or unpasteurized milk consumption was observed among the sub-analysis of cases reported in Washington (Table 3).
Table 3.
Exposure to chitterlings by race and age group, within seven days prior to illness onset among yersiniosis cases in Illinois and Washington, 2005–2011
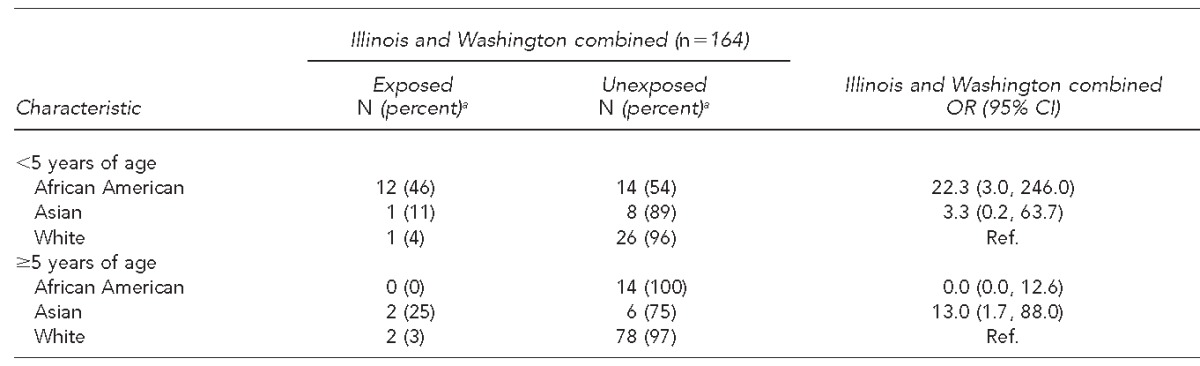
aPercentages are row percentages.
OR = odds ratio
CI = confidence interval
Ref. = reference group
DISCUSSION
Analysis of yersiniosis surveillance data from these nine passive surveillance states demonstrated a large decline in incidence among African American children <5 years of age and an increasing trend for white adults and people of Hispanic ethnicity. The decline in incidence among African American children was consistent with the FoodNet report; however, unlike FoodNet, annual incidence was not the highest among Asian children during the period 2007–2009. There was no significant trend observed in other age and race groups and in the overall incidence during this period. These data also revealed variations in seasonal trends by race.
The methods used and the findings of this analysis had some similarities and differences with those of the recent FoodNet report on yersiniosis.15 In both reports, incidence rates were calculated using population estimates from the U.S. Census Bureau. The overall annual incidence rates in the FoodNet catchments population were higher (0.5 per 100,000 population) compared with our study population (0.16 per 100,000 population). Variation in the time period covered in these reports could partially explain this difference. FoodNet reported data for the period 1996–2009. Higher incidence in earlier years of the FoodNet surveillance likely resulted in higher overall annual incidence compared with our study, which covered a later and shorter time period (2005–2011). Also, the active surveillance of FoodNet is likely more efficient in detecting cases than the passive surveillance in these states.
Consistent with the recently published report from FoodNet,15 we observed a higher average annual incidence of yersiniosis among African American and Asian children compared with white children. Exposure to chitterlings, other raw or undercooked pork products, and possible cross-contamination from improper handwashing by caretakers preparing chitterlings before or while caring for infants have been associated with outbreaks and sporadic cases of yersiniosis in this vulnerable age group.5,6,9,19 Susceptibility of the infants to indirect transmission and subsequent infection with Yersinia enterocolitica might be due to their lack of prior exposure and lack of specific immunity against the pathogen.20 We are not aware of any specific yersiniosis prevention interventions in these nine states that may have resulted in the reduction of cases in their populations.
The incidence of yersiniosis has been decreasing in the United States. However, the reasons for this decrease are not clear.21 A factor that may explain the 56% decline in incidence among African American children in this study population might include an overall increase in awareness about the importance of hand hygiene,22 especially during the winter months, as this time period overlapped with several highly publicized influenza seasons. This time period also included the influenza pandemic of 2009, which was known as “swine flu” and erroneously linked by some media reports to eating pork products. These influenza seasons included substantial media attention to hand hygiene, which overlapped with the time of year when chitterlings are more likely to be consumed. Therefore, at least theoretically, African Americans might have paid increased attention to hand hygiene, including during chitterlings preparation, or avoidance of pork products entirely for a period of time, thus reducing the likelihood of transmission to their infants. Another aspect that may have contributed to a decline in cases is the availability and possible increase in the use of precooked/preboiled chitterlings, which is safer than raw chitterlings and is recommended by the U.S. Department of Agriculture Food Safety and Inspection Service.23 In Georgia, a high-incidence state that participates in FoodNet, the health department also promoted precooked/preboiled chitterlings as a yersiniosis prevention activity.24 Given that the decline we observed is important and substantial but not readily explainable, further research (e.g., case-control studies) should be conducted to explore possible risk factors and related practices.
The annual incidence of yersiniosis varied across race and age groups. Although the average incidence was similar in adults of different races, we observed an increasing trend among white adults that was not statistically significant at a=0.05. In contrast with the decreasing trend among African American children <5 years of age, we observed no significant decrease among children <5 years of age from other races. Future studies should explore possible preventable risk factors for yersiniosis beyond exposure to chitterlings, as even among high-risk African American children <5 years of age—the group with the highest risk—chitterlings exposure did not account for the majority of cases. While pork exposure in general has been documented as a risk factor, in the United States and in Germany,19,25 a recent case-control study conducted in Sweden among children <7 years of age identified contact with domestic animals, in particular dogs and cats, and pacifier use as risk factors.26 Epidemiologic studies identifying risk factors for children in the United States are needed, as chitterlings and pork exposure may not fully explain infection and may lead to useful targeted prevention efforts. Among foodborne diseases, yersiniosis prevention receives relatively little attention.
Upon examination of seasonality, variability by race was apparent. Similar to FoodNet data,15 we observed increased reports of cases in winter months among African Americans. However, we also observed an increase in cases among white people during April–June. While the winter peak coincides with various holidays, the April–June peak may be related to Easter holidays, as the Easter Sundays of five of these seven years fell in April and the other two were during the last week of March. Among the African American population, chitterlings are a traditional food for Thanksgiving and Christmas, while ham is a common traditional food for the Easter dinner in the United States. Therefore, these data demonstrate that educational efforts might have greater impact if emphasized during these times of the year, just as health departments often promote food safety for cooking turkey and barbecued meats during Thanksgiving and summer months.
Limitations
This study was subject to several limitations. For one, we may have underestimated the true burden of disease. A study of stool isolates from children in Atlanta, Georgia, reported Yersinia enterocolitica to be the second most common bacteria isolated from stool in this age group.27 As cultural isolation of Yersinia enterocolitica from stool is hampered by its slow growth and by overgrowth of other fecal flora, a special selective media is required.3 However, to lower costs, routine stool culture in laboratories may not include this medium or may only be included during fall or winter for at-risk populations.28,29 For example, in evaluating a convenience sample of six hospital laboratories in Chicago, Illinois, through telephone interviews, only one reported that it includes a special medium to grow Yersinia enterocolitica with its routine stool culture procedure. Consequently, many cases may remain undiagnosed and unreported. Because only laboratory-confirmed cases are reported to the surveillance system, people with mild illness and those without access to health care might have been missed in this report.
Incomplete reporting of race/ethnicity data was another limitation of this study. Race information was missing for 26% of cases in the nine states and 21% of cases in the FoodNet report.15 The high percentage of missing data for race/ethnicity in these states might have skewed the overall interpretation of yersiniosis distribution. Given the large proportion of missing race/ethnicity data in different states and the variability in data by race demonstrated in this study and by FoodNet, state and local health departments should be vigilant in obtaining this information from health-care providers and laboratories, including considering requiring the addition of entry of these data elements in electronic reporting systems.
The findings are not generalizable to the entire United States, in part because no state from the mid-Atlantic or Northeast region was included in this analysis. Despite these limitations, these data were derived from nine states representing 20% of the U.S. population and provide useful comparisons of passive surveillance with FoodNet. When collectively analyzed, these reports from 19 states (nine from this study and 10 from the FoodNet report) provide a broader view of yersiniosis in the United States and are an important contribution toward the understanding of yersiniosis epidemiology and guiding public health action toward reducing incidence.
CONCLUSIONS
This multistate analysis of yersiniosis reveals that although incidence has been decreasing among African American children <5 years of age, such declines have not been observed in other race and age groups. Because of the consistent seasonality of higher infection rate, future efforts to prevent yersiniosis should consider heightened educational campaigns targeted during November–January and March–April. Prevention research should consider racial/ethnic differences that may play a role, perhaps related to food recipes or food handling differences. Studies informing optimal messaging are needed, as the risk factors for sporadic disease in the United States are not well characterized. Increased attention to completeness of reporting race/ethnicity data is critical to thoughtful hypothesis generation, and epidemiologic studies of risk factors among reported cases are needed to more precisely identify the risk factors.
Footnotes
This project involved de-identified public health surveillance data and was determined not to be research, so the project did not undergo institutional review board review.
The authors acknowledge the support of the participating state health departments in Arizona, Illinois, Michigan, Missouri, Nebraska, North Carolina, South Carolina, Washington, and Wisconsin for sharing their data, and thank Del Williams, PhD, head of the Surveillance, Communicable Disease Branch, North Carolina Department of Health and Human Services, for extracting the surveillance data for North Carolina.
REFERENCES
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen J, Powderly WG, Opal SM. Infectious diseases. 3rd ed. Edinburgh: Mosby/Elsevier; 2010. [Google Scholar]
- 3.Smego RA, Frean J, Koornhof HJ. Yersiniosis I: microbiological and clinicoepidemiological aspects of plague and non-plague yersinia infections. Eur J Clin Microbiol Infect Dis. 1999;18:1–15. doi: 10.1007/s100960050219. [DOI] [PubMed] [Google Scholar]
- 4.Bottone EJ. Yersinia enterocolitica: the charisma continues. Clin Microbiol Rev. 1997;10:257–76. doi: 10.1128/cmr.10.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stolk-Engelaar VM, Hoogkamp-Korstanje JA. Clinical presentation and diagnosis of gastrointestinal infections by Yersinia enterocolitica in 261 Dutch patients. Scand J Infect Dis. 1996;28:571–5. doi: 10.3109/00365549609037963. [DOI] [PubMed] [Google Scholar]
- 6.Rosner BM, Stark K, Werber D. Epidemiology of reported Yersinia enterocolitica infections in Germany, 2001–2008. BMC Public Health. 2010;10:337. doi: 10.1186/1471-2458-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Haq NM, Asmar BI, Abuhammour WM, Brown WJ. Yersinia enterocolitica infection in children. Pediatr Infect Dis J. 2000;19:954–8. doi: 10.1097/00006454-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 8.alMohsen I, Luedtke G, English BK. Invasive infections caused by Yersinia enterocolitica in infants. Pediatr Infect Dis J. 1997;16:253–5. doi: 10.1097/00006454-199702000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Adamkiewicz TV, Berkovitch M, Krishnan C, Polsinelli C, Kermack D, Olivieri NF. Infection due to Yersinia enterocolitica in a series of patients with beta-thalassemia: incidence and predisposing factors. Clin Infect Dis. 1998;27:1362–6. doi: 10.1086/515025. [DOI] [PubMed] [Google Scholar]
- 10.Jones TF. From pig to pacifier: chitterling-associated yersiniosis outbreak among black infants. Emerg Infect Dis. 2003;9:1007–9. doi: 10.3201/eid0908.030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longenberger AH, Gronostaj MP, Yee GY, Johnson LM, Lando JF, Voorhees RE, et al. Yersinia enterocolitica infections associated with improperly pasteurized milk products: southwest Pennsylvania, March–August, 2011. Epidemiol Infect. 2014;142:1640–50. doi: 10.1017/S0950268813002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babic-Erceg A, Klismanic Z, Erceg M, Tandara D, Smoljanovic M. An outbreak of Yersinia enterocolitica O:3 infections on an oil tanker. Eur J Epidemiol. 2003;18:1159–61. doi: 10.1023/b:ejep.0000006631.59644.1d. [DOI] [PubMed] [Google Scholar]
- 13.Ackers ML, Schoenfeld S, Markman J, Smith MG, Nicholson MA, DeWitt W, et al. An outbreak of Yersinia enterocolitica O:8 infections associated with pasteurized milk. J Infect Dis. 2000;181:1834–7. doi: 10.1086/315436. [DOI] [PubMed] [Google Scholar]
- 14.Tacket CO, Narain JP, Sattin R, Lofgren JP, Konigsberg C, Jr, Rendtorff RC, et al. A multistate outbreak of infections caused by Yersinia enterocolitica transmitted by pasteurized milk. JAMA. 1984;251:483–6. [PubMed] [Google Scholar]
- 15.Ong KL, Gould LH, Chen DL, Jones TF, Scheftel J, Webb TH, et al. Changing epidemiology of Yersinia enterocolitica infections: markedly decreased rates in young black children, Foodborne Diseases Active Surveillance Network (FoodNet), 1996–2009. Clin Infect Dis. 2012;54(Suppl 5):S385–90. doi: 10.1093/cid/cis053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Census Bureau (US) Population census 2010. Updated 2011 [cited 2013 May 15] Available from: URL: http://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t.
- 17.SAS Institute, Inc. Cary (NC): SAS Institute, Inc.; 2005. SAS®: Version 9.1.3. [Google Scholar]
- 18.Microsoft Corp. Redmond (WA): Microsoft Corp.; 2010. Microsoft® Excel®: Version 2010. [Google Scholar]
- 19.Rosner BM, Stark K, Hohle M, Werber D. Risk factors for sporadic Yersinia enterocolitica infections, Germany 2009–2010. Epidemiol Infect. 2012;140:1738–47. doi: 10.1017/S0950268811002664. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MB. Etiology and mechanisms of acute infectious diarrhea in infants in the United States. J Pediatr. 1991;118:S34–9. doi: 10.1016/s0022-3476(05)81423-4. [DOI] [PubMed] [Google Scholar]
- 21.Vugia D, Hadler J, Chaves S, Blythe D, Smith K, Morse D, et al. Preliminary FoodNet data on the incidence of foodborne illnesses—selected sites, United States, 2002. MMWR Morb Mortal Wkly Rep. 2003;52(15):340–3. [PubMed] [Google Scholar]
- 22.American Society of Microbiology. Clean hands campaign results best ever [cited 2013 Jun 20] Available from: URL: http://www.microbemagazine.org/index.php?option=com_content&view=article&id=3055:clean-hands-campaign-results-best-ever&catid=701&Itemid=905.
- 23.Department of Agriculture (US), Food Safety and Inspection Service. Yersiniosis and chitterlings: tips to protect you and those you care for from foodborne illness [cited 2014 Dec 5] Available from: URL: http://www.fsis.usda.gov/wps/portal/fsis/topics/food-safety-education/get-answers/food-safety-fact-sheets/foodborne-illness-and-disease/yersiniosis-and-chitterlings/ct_index.
- 24.Georgia Division of Public Health. Georgia epidemiology report: healthcare providers: be alert for yersiniosis. 1998 [cited 2013 Jun 5] Available from: URL: http://health.state.ga.us/pdfs/epi/gers/ger0998.pdf.
- 25.Tauxe RV, Vandepitte J, Wauters G, Martin SM, Goossens V, De Mol P, et al. Yersinia enterocolitica infections and pork: the missing link. Lancet. 1987;1:1129–32. doi: 10.1016/s0140-6736(87)91683-7. [DOI] [PubMed] [Google Scholar]
- 26.Boqvist S, Pettersson H, Svensson A, Andersson Y. Sources of sporadic Yersinia enterocolitica infection in children in Sweden, 2004: a case-control study. Epidemiol Infect. 2009;137:897–905. doi: 10.1017/S0950268808001209. [DOI] [PubMed] [Google Scholar]
- 27.Metchock B, Lonsway DR, Carter GP, Lee LA, McGowan JE., Jr Yersinia enterocolitica: a frequent seasonal stool isolate from children at an urban hospital in the southeast United States. J Clin Microbiol. 1991;29:2868–9. doi: 10.1128/jcm.29.12.2868-2869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (US) Yersinia [cited 2013 Jun 20] Available from: URL: http://www.cdc.gov/nczved/divisions/dfbmd/diseases/yersinia.
- 29.Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331–51. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]


