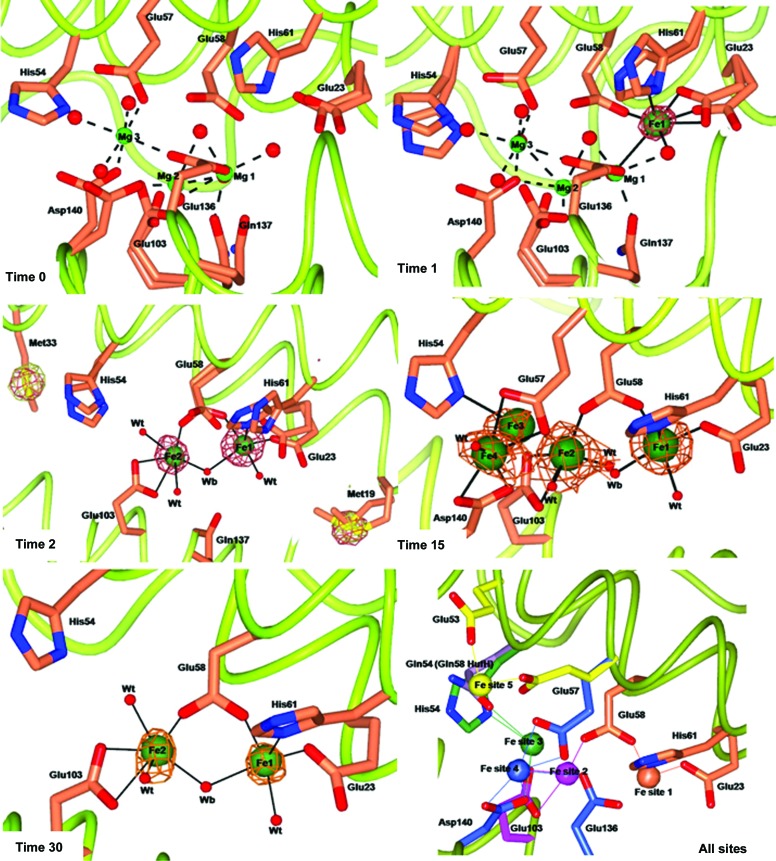
Figure 1.
Effect of different exposure times to Mohr’s salt on metal ion binding to wt-RcMf crystals. wt-RcMf crystallized with MgCl2 (time 0 structure) and iron binding at 1, 2, 15 and 30 min exposure. Magnesium and iron ions are represented by light and dark green spheres, respectively (arbitrary radius). The anomalous difference map contoured at 5.0σ, shown as copper wire, is superimposed on the iron ions. To highlight the speciation capability of the measurements, the time 2 panel shows the side chains of Met19 and Met33 superimposed with the two anomalous maps obtained at the Fe K edge (copper wire) and below the Fe K edge (white wire). The Fourier maps corresponding to other atoms have been omitted for clarity. The amino acids involved in iron binding are shown as sticks. Double conformations of the side chains of residues involved in metal ion binding are also shown. Water molecules are shown as small red spheres. Mg2+ coordination bonds are shown as dashed lines. Fe2+ coordination bonds are shown as continuous lines. The last panel displays a scheme summarizing the positions of the five Fe2+-binding sites observed in average RcMf (and the H54Q variant). Protein ligands are represented by sticks of different colours. Fe ions are represented as spheres colour-coded by their protein ligands. Ligands are as follows. Fe site 1 (coral): Glu23, Glu58 (bridging ligand) and His61. Fe site 2 (magenta): Glu58 (bridging ligand) and Glu103. Fe site 3 (green) involves His54 in RcMf or Gln54 in RcMf H54Q and also Glu57 and Glu103 (bonds not shown for clarity). Fe site 4 (blue) is located on the subunit surface facing the ferritin cavity and involves binding to Glu57, Glu136 and Asp140. The position of His54 is structurally coincident with Gln58 of human H ferritin, shown as lilac sticks. Fe site 5 (yellow) is found in the ferritin H54Q variant (see Fig. 3 ▶, time 1): Glu53 and Glu57 assume alternative conformations with respect to wt-RcMf.