Table 1. Statistics of diffraction data and structure refinement.
Values in parentheses are for the highest resolution shell.
| Native (PDB entry 4ts4) | Native + 10-FDDF (PDB entry 4tt8) | Native + THF (PDB entry 4qpd) | Native + formate (PDB entry 4r8v) | Y200A mutant (PDB entry 4qpc) | Y200A mutant + 10-FDDF (PDB entry 4tts) | |
|---|---|---|---|---|---|---|
| Data collection | ||||||
| Wavelength () | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Temperature (K) | 110 | 110 | 110 | 110 | 110 | 110 |
| Space group | P21212 | P21212 | P212121 | P21212 | P21212 | P21212 |
| Unit-cell parameters () | ||||||
| a | 104.67 | 104.08 | 54.5 | 103.98 | 104.46 | 103.64 |
| b | 53.88 | 52.69 | 100.42 | 51.98 | 53.96 | 54.38 |
| c | 60.63 | 60.5 | 122.52 | 59.94 | 60.88 | 61.01 |
| Resolution () | 301.75 (1.811.75) | 302.30 (2.382.30) | 302.10 (2.182.10) | 302.20 (2.282.20) | 301.90 (1.971.90) | 302.00 (2.072.00) |
| Completeness (%) | 99.9 (99.9) | 100 (100) | 98.8 (89.0) | 99.6 (99.9) | 92.6 (99.8) | 97.4 (100) |
| Multiplicity | 6.9 (6.5) | 5.6 (5.6) | 5.2 (3.2) | 5.5 (5.6) | 5.6 (5.7) | 6.0 (6.4) |
| I/(I) | 33.8 (4.0) | 15.8 (4.3) | 18.9 (2.1) | 20.5 (3.7) | 14.1 (5.0) | 12.6 (4.3) |
| R merge † (%) | 5.7 (44.0) | 9.8 (43.9) | 7.9 (43.7) | 8.2 (48.6) | 10 (35.2) | 9.7 (33.2) |
| Refinement | ||||||
| Resolution range () | 301.75 | 302.30 | 302.10 | 302.20 | 301.90 | 302.00 |
| R work ‡/R free § (%) | 18.0/20.7 | 18.0/22.4 | 18.5/23.3 | 19.3/23.6 | 20.4/22.5 | 20.1/24.5 |
| No. of atoms | ||||||
| Protein | 2415 | 2415 | 4830 | 2415 | 2408 | 2408 |
| Ligand/ion | 48 | 110 | 24 | 34 | ||
| Water molecules | 332 | 162 | 432 | 85 | 345 | 291 |
| B factors (2) | ||||||
| Protein | 30.1 | 32.8 | 34.3 | 39.8 | 30.0 | 20.9 |
| Ligand/ion | 45.8 | 40.6 | 57.4 | 37.8 | ||
| Water molecules | 37.0 | 34.2 | 36.7 | 36.7 | 36.3 | 25.0 |
| R.m.s. deviations | ||||||
| Bond lengths () | 0.007 | 0.004 | 0.006 | 0.007 | 0.006 | 0.008 |
| Bond angles () | 1.300 | 0.968 | 1.010 | 1.114 | 1.053 | 1.260 |
R
merge = 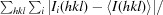
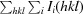 , where I
i(hkl) is the ith measurement and I(hkl) is the weighted mean of all measurements of I(hkl).
, where I
i(hkl) is the ith measurement and I(hkl) is the weighted mean of all measurements of I(hkl).
R
work = 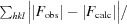
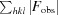 , where F
obs and F
calc are the observed and calculated structure-factor amplitudes of reflection hkl, respectively.
, where F
obs and F
calc are the observed and calculated structure-factor amplitudes of reflection hkl, respectively.
R free is calculated as for R work but with a randomly chosen 5% of reflections that were omitted from refinement.
