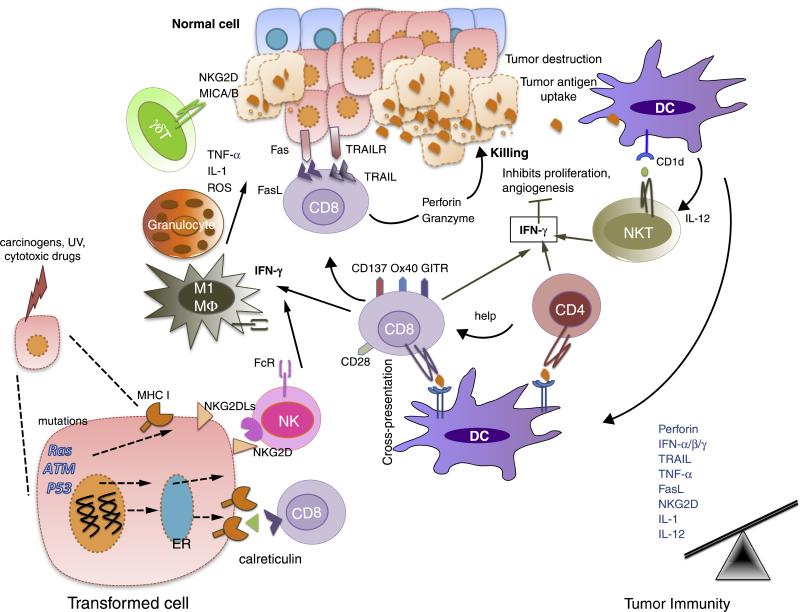
Figure 2.
Elimination is a phase of cancer immunoediting where both the innate and adaptive immune system together detect and destroy early tumors before they become clinically visible. Normal cells (blue) are transformed into tumor cells by carcinogens and other genotoxic insults along with the failure of intrinsic tumor suppressor mechanisms (e.g. p53, ATM). These tumor cells express stress-induced molecules such as surface calreticulin, tumor antigens in context of MHC class I molecules, and/or NKG2D ligands recognized by CD8+ effector cells and NK cells, respectively. DCs can also take up and cross-present tumor antigens to T cells including NKT cells (glycolipid antigens presenting via CD1d). These activated effector cells release IFN-γ that can mediate anti-tumor effects by inhibiting tumor cell proliferation and angiogenesis. CD8+ T cells can induce tumor cell apoptosis by interacting with Fas and TRAIL receptors on tumor cells, or by secreting perforin and granzymes. Effector T cells express co-stimulatory molecules such as CD28, CD137, GITR, OX40 that enhance their proliferation and survival. γδ T cells can also recognize and kill tumors expressing NKG2D ligands (MICA/B in humans). Innate immune cells such as macrophages (M1) and granulocytes also contribute to anti-tumor immunity by secreting TNF-α, IL-1, IL-12 and ROS. In the Elimination phase, the balance is towards anti-tumor immunity due to an increase in expression of tumor antigens, MHC class I, Fas and TRAIL receptor on tumor cells and perforin, granzymes, IFN-α/β/γ, IL-1, IL-12, TNF-α in the tumor microenvironment.