Figure 2.
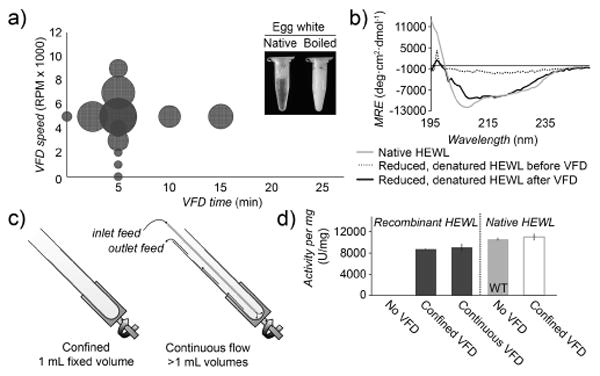
Determination of secondary structure and activity of hen egg white lysozyme (HEWL) processed by VFD. a) Lysozyme activity per mg protein following VFD processing of boiled egg white (90 °C, 20 min) at a fixed VFD speed of 5 krpm, or a fixed 5 min refolding time. The relative circle size represents the lysozyme activity with larger circles representing higher recovered activities, plotted as a function of VFD time and speed. b) CD spectra of recombinantly expressed, reduced, and denatured HEWL before (dotted) and after (dark gray) VFD refolding. c) In continuous flow mode, the protein solution is introduced through a thin, hollow metal tube to the bottom of the sample tube, and the folded protein can be collected at the top of the VFD. d) Lysozyme activity per mg protein following VFD refolding of recombinantly expressed HEWL and native HEWL. Under these conditions, VFD treatment of recombinant lysozyme recovered over 82% of activity in both confined and continuous flow modes, compared to wild-type protein (WT). VFD treatment of wild-type, active lysozyme isolated from eggs does not adversely affect its activity (white). Throughout this report, error bars indicate standard deviation (n = 3).
