Figure 3.
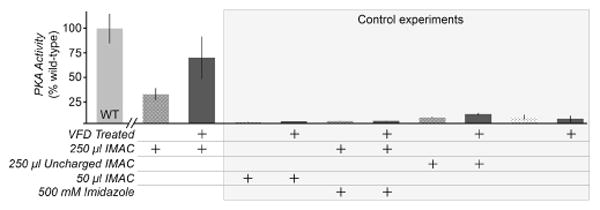
Determination of secondary structure and activity of caveolin-ΔTM processed by VFD. a) Circular dichroism (CD) spectra of caveolin-ΔTM following VFD induced refolding or conventional dialysis. b) Turbidity of caveolin-ΔTM, measured by the fractional absorbance at 600 nm compared to the untreated sample also at 600 nm. The arrow indicates absorbance of caveolin-ΔTM following 4-days of dialysis. Both 30 min of VFD-based refolding and 96 h of dialysis significantly decrease the turbidity of the protein solution. c) Binding of caveolin-ΔTM to gp41 determined by ELISA using untreated, VFD-treated, and conventionally refolded by dialysis. All other conditions and buffers remained identical. Although some binding occurs without VFD treatment, caveolin-ΔTM binds with greater affinity after refolding by VFD.
