Figure 4.
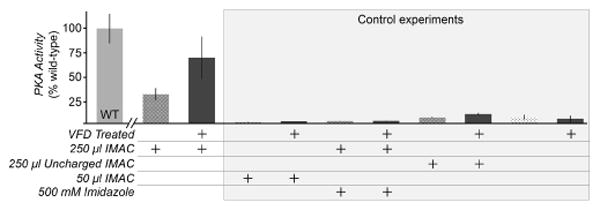
Facilitating VFD refolding of PKA by pre-binding the His6-taggged protein to IMAC resin. 1.7 mg PKA was pre-incubated with 250 or 50 μl IMAC resin in 6 M guanidine-HCl prior to dilution to 1 M guanidine-HCl and VFD treatment. Excess PKA was removed by a low imidazole (1 mM) wash buffer. The PKA activity per μg of protein was measured by a nicotinamide adenine dinucleotide (NADH) enzyme-linked assay, and shown here as a percentage of untreated, wild-type PKA activity (WT). Dark gray bars denote samples that are VFD-treated. In this assay, the consumption of ATP by PKA catalysis results in a lactate dehydrogenase-coupled decrease in the levels of NADH, monitored through measuring absorbance at 340 nm. The control experiments included low quantities of resin (50 μl IMAC), and two controls for non-specific binding to the resin in the absence of the Ni2+-His6 tag interaction (500 mM imidazole added and uncharged IMAC resin lacking Ni2+). Imidazole was diluted to 50 mM before kinase assay.
