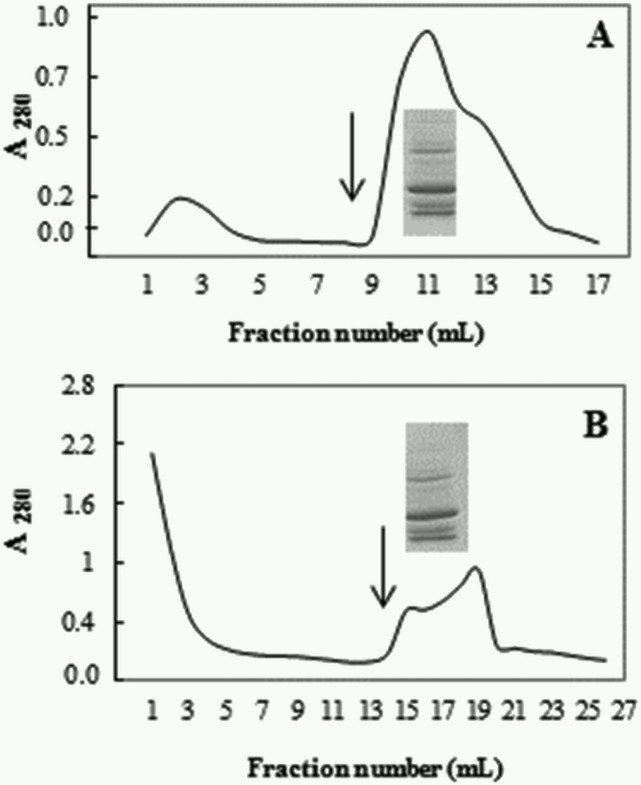
Fig 4. Purification of Blad-oligomer by chitin-affinity chromatography.
(A,B) Pure Blad-containing protein (1.2 absorbance units; A) or the total globulin fraction from 8-days germinated Lupinus cotyledons (10 absorbance units; B), respectively, were loaded into a chitin column previously equilibrated with 50 mM Tris-HCl buffer, pH 7.5. The column was washed and the bound proteins eluted with 0.05 N HCl (beginning of elution is marked with a vertical arrow). One mL fractions were collected. SDS-PAGE analysis of the polypeptide patterns of Blad-oligomer by the standard, extensive procedure or purified by the single step, chitin-affinity chromatography procedure are illustrated in the figure.