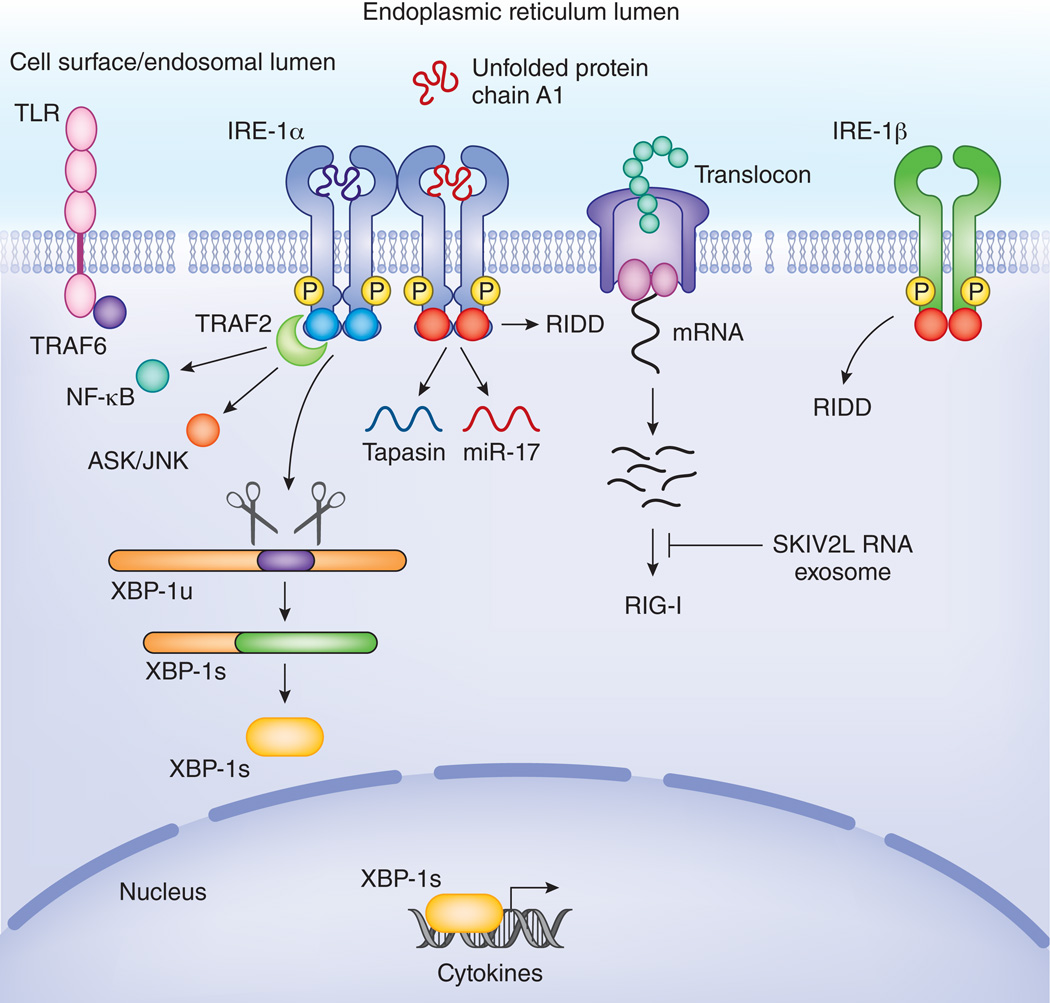
Figure 3. IRE1 intersects with inflammatory pathways.
TLRs stimulate IRE1 mediated XBP-1 splicing in a TRAF6 and NOX2 dependent manner. XBP-1s binds at the promoter of several cytokines and is needed for optimal cytokine expression. Phosphorylated IRE1 interacts via TRAF2 with the IKK complex and with ASK/JNK. In this way, IRE1 controls activation of NF-κB and AP-1, 2 major inflammatory transcription factors. IRE1 also serves as a host defense receptor, surveying the lumen of the ER for pathogens. In response to cholera toxin, IRE1 is activated and starts degrading mRNAs at the translocon complex. This generates several short RNA stretches that ligate RIG-I and trigger IFN-α via RIG-I. These RNA species can in turn be degraded by the SKIV2L exosome to prevent inappropriate inflammatory responses. RIDD also leads to degradation of specific mRNAs like that encoding tapasin as well as microRNAs such as miR-17. Degradation of tapasin and other components of the MHC-I loading machinery interfere with antigen presentation. Degradation of miR17, leads to degradation of TXNIP and subsequent stabilization of the NLRP3 inflammasome which causes enhanced IL-1β release. In addition to IRE-1α, a second isoform called IRE-1β is specifically expressed in epithelial cells lining mucosal interfaces, such as bronchi or the colon. Its RIDD function appears to predominate its capacity to splice XBP-1. This might endow IRE-1β with an enhanced capacity to exert immune surveillance and control the interface between the host and microbes present in the colon or airways.