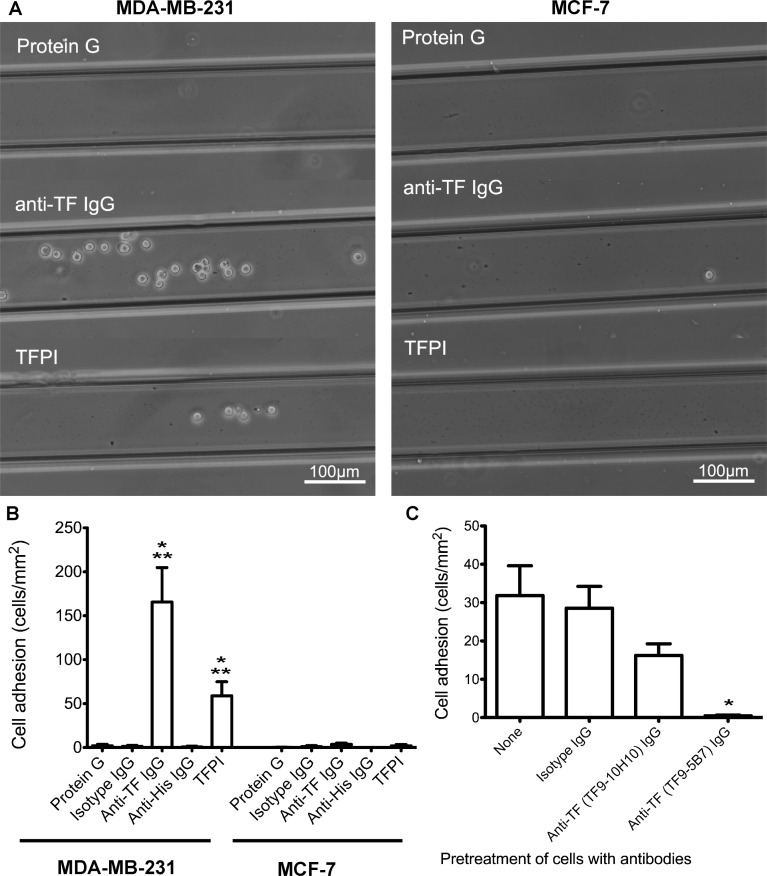
Fig 4. Adhesion of tumor cells to protein-immobilized microfluidic channels under low shear (0.35dyn/cm2).
Microfluidic channels were incubated with Protein G (100μg/ml), then anti-TF IgG (100μg/ml), or an anti-His antibody (100μg/ml) followed by TFPI (100μg/ml). Isotype IgG (100μg/ml) and anti-His IgG (100μg/ml) antibodies were used as negative control for anti-TF IgG and TFPI respectively. Tumor cells (1x106cells/mL, pre-treated with 10nM FVIIa and 10nM FX for TFPI-coated channels) were introduced into the channels at 0.35dyn/cm2 for 30 minutes, and non-specifically adhered cells were removed at 2.0dyn/cm2. The entire channel was imaged to quantify the number of adherent cells. A. Representative bright field images of adherent tumor cells on channels immobilized with Protein G (negative control), anti-TF IgG and TFPI showing that more MDA-MB-231 than MCF-7 cells were bound to both anti-TF IgG- and TFPI-coated channels. B. The number of adherent cells was counted and normalized by the channel area. MDA-MB-231 showed significantly higher adhesion to TFPI- and anti-TF IgG-coated channels than MCF-7 (* p < 0.05, n = 4 for anti-TF IgG, n = 3 for TFPI). Significantly more MDA-MB-231 bound to TFPI- and anti-TF IgG-coated channels than negative controls (** p<0.05). C. MDA-MB-231 cells were pretreated with 50μg/ml anti-TF IgG (TF9-5B7 which blocks FVIIa binding to TF, or TF9-10H10 which does not block FVIIa binding to TF). The positive control had no antibody pretreatment, and isotype IgG pretreatment (50μg/ml) was used as a negative control. Blocking FVIIa binding to TF with TF9-5B7 antibody significantly decreased adhesion to TFPI-coated channels (* p < 0.05, n = 4). The observed decrease in MDA-MB-231 adhesion with the TF9-10H10 antibody, albeit not significant with this stringent statistical test, could be due to steric hindrance of TFPI binding to the TF/FVIIa/FXa complex on the tumor cells.