Abstract
BACKGROUND/OBJECTIVES
Rheumatoid arthritis (RA) is associated with an excess mortality from cardiovascular disease which is likely attributed to an atherogenic lipid profile. Among nutritional factors vitamin K has been recently focused as a pivotal nutrient in improvement of lipid related markers. Thus, this study was designed to determine the effects of vitamin K on lipid profile in this disease.
SUBJECTS/METHODS
Fifty eight patients with definitive RA were participated in the present double blind placebo controlled study. They were randomly allocated into two groups to receive vitamin K1 as phylloquinone [10 mg/day] (n = 30) or placebo pills (n = 28), for eight weeks. In order to control the effects of probable confounders dietary intakes, anthropometric measurements including weight and height, clinical status using disease activity score-28 (DAS-28), physical activity and anxiety status were evaluated at baseline. Moreover, serum levels of lipid related markers including total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and triglyceride (TG) were measured at baseline and at the end of intervention.
RESULTS
There were no significant differences between the two groups regarding any of the baseline characteristics. After adjusting for some relevant confounders, in comparison between two groups, we observed no significant changes in lipid related markers at the end of intervention. Also, there was no significant difference between before and after intervention values within groups (P > 0.05).
CONCLUSIONS
Function of vitamin K1 in lipid profile modification remains still controversial. This study showed that vitamin K1 has no effect on lipid profile in women with rheumatoid arthritis. Further studies with a longer follow-up are required to determine the effects of vitamin K on atherogenic lipid profile.
Keywords: Rheumatoid arthritis, vitamin K1, phylloquinone, lipid profile
INTRODUCTION
Rheumatoid arthritis (RA) which is outlined as the most common cause of polyarthritis has increasingly affected people through the last decade [1]. Despite of a remarkable increase in prevalence of this disease in the United States [2], the growing incidence rate of RA in the other developing countries such as Iran has been noticeable as one of the major challenges for public health [3].
Cardiovascular diseases and dislipidemia are considered as the leading causes involved in the mortality of RA [4,5]. There are several lines of evidence imply an atherogenic lipid profile in patients with rheumatoid arthritis [4,6,7]. In accordance of their findings, high levels of low-density lipoprotein cholesterol (LDL-C), total cholesterol and low level of high-density lipoprotein cholesterol (HDL-C) are correlated with the disease activity [4]. It is supposed that increased levels of pro-inflammatory mediators like TNFα and CRP may possibly account for this atherogenic lipid profile [4]. Thus, trying to relieve inflammation in inflammatory disorders such as RA might lead to a great improvement of lipid profile.
Among dietary factors, vitamin K has been recently considered to have an important role in controlling inflammation and consequently in cardiovascular risk factors [1]. Previous studies investigating the effect of vitamin K on inflammation found it as a new immunomodulating factor [8,9].
Furthermore, vitamin K is thought to have a possible direct function in cardioprotection [10]. This putative effect of vitamin K in atherosclerosis is explained through a few pathways. Calcification of soft tissues and consequently vessel rupture also inhibition of fat deposition in marrow cells are blocked in presence of sufficient amount of vitamin K [10,11].
Former studies reported a favorable effect of vitamin K on plasma total cholesterol, lipid peroxidation and ester-cholesterol deposition in the aorta of hypercholesterolemic rabbits [12]. These results were in keeping with those obtained in other investigations in rats and patients on continuous ambulatory peritoneal dialysis which confirmed a lipid lowering effect of vitamin K [11,13]. However, Kristensen et al. [14] have not found similar results in postmenopausal women and surprisingly reported undesirable effects of vitamin K on blood lipids.
We hypothesized that vitamin K supplementation might have lipid lowering effects in patients with RA. Therefore, regarding this discrepancy in previous findings and high prevalence of mortality from rheumatoid arthritis due to cardiovascular risk factors, we aimed to examine the effects of vitamin K1 on lipid profile after 8 weeks intervention in women with rheumatoid arthritis.
SUBJECTS AND METHODS
Study population
This randomized, double blind, placebo controlled trial was conducted on sixty four eligible women with RA accepting to participate. Patients were recruited from the outpatient rheumatology clinic of Imam Reza Hospital, Tabriz, Iran, July to December 2012.
Study eligibility criteria included being 20-50 years, documentation of (RA) regarding to the American College of Rheumatology (ACR, 2010) classification for RA [15] which is diagnosed by rheumatology specialist, absence of chronic diseases, i.e., diabetes, liver or kidney disorders, malabsorption and cardiovascular disease, thyroid disorders, coagulation abnormality, patients with prothrombin time < 10s, hyperprolactinemia, Cushing' syndrome, super obesity (Body Mass Index > 40), cancer, high blood pressure, not taking hormonal therapy, contraceptive pills, antibiotics, antiepileptic drugs or other dietary supplements during the past 3 months. Being pregnant, lactate, postmenopause, being in severe stage of rheumatoid arthritis (Disease Activity Score-28 > 5.1), smoking, lifestyle change and drug compliance less than 75% were the study exclusion criteria.
Study protocol
List of patients with rheumatoid arthritis who referred to rheumatology clinic of Imam Reza Hospital, Tabriz, Iran, enabled us to have a preliminary screening by telephone and following it an orientation meeting was held for the volunteers. The meeting provided a comprehensive description about the purpose and protocol of study. Then, the written consent form was filled out by the participants. The demographic characteristics and anthropometric measurements were also obtained.
Anthropometric measurements
Weight (to the nearest 0.1 kg) and height (to the nearest 0.5 cm) were measured in a standard position using a scale (Seca, Germany) and a wall-meter respectively. Finally, body mass index (BMI) was calculated for each patient with the usual formula: weight (kg) / height2 (m2).
Assessment of anxiety level
Given an important role of anxiety in exacerbation of symptoms in RA, the participants' anxiety status was measured by the State-Trait Anxiety Inventory (STAI-Y) scale before intervention. This standard scale has two 20-question sections. The first part is designed to assay the state anxiety which evaluates the current state of anxiety and their feeling at the moment and the second part focuses on the trait anxiety which includes general and stable states of anxiety. All of these data were collected by face to face interview. Finally patients were classified as having no or minimum, low, intermediate or severe state and trait anxiety regarding their scores from STAI-Y questionnaires [16].
Assessment of physical activity level
Short International Physical Activity Questionnaire (IPAQ) was used to evaluate participants' physical activity level (low, moderate and high). This questionnaire includes 7 questions and its validity and reliability has been investigated in previous studies [17,18]. Physical activity of each subject was measured considering the energy requirements defined in METS (metabolic equivalent). These following values are used for the analysis of IPAQ data: walking = 3.3 METs, moderate physical activity = 4.0 METs and vigorous physical activity = 8.0 METs. After calculation Metminutes/week (= MET level × minutes of activity/day × days per week), participants were classified in three groups (low, moderate and high physical Activity). High intensity activity was considered for vigorous activity on at least 3 days of a week, with at least 1500 MET-minutes/week or at least five days of combination of walking, moderate or vigorous intensity activities with at least 3000 MET-minutes/week. Moderate intensity activity was considered for five or more days of any combination of walking, moderate-intensity and vigorous intensity activities achieving of at least 600 MET-minutes/week and if no activity was reported or it wasn't enough to consider in two mentioned classes, it was classified as low physical activity [19].
Assessment of disease activity score
Disease Activity Score (DAS-28) is a quantitative measure to describe patient status in rheumatic diseases was evaluated by rheumatology specialist through the formula with 3 variables [DAS = 0.56 (tender joint count)2 + 0.28 (swollen joint count)2 + 0.36 × Ln (CRP + 1) × 1.10 + 1.15]. DAS-28 scores represent remission (< 2.6), mild (< 3.2), moderate (< 5.1) and severe (> 5.1) disease activity [20].
Assessment of nutritional status
Participants' nutritional status was assayed by a three-day food records. Subjects were advised to record all foods and beverages consumed throughout the day for two days of the week and one day of the weekend. According to the guideline of food portion size [21], amount of each dietary item they consumed was expressed in gram. Then these data were analyzed using Nutritionist IV software (First Databank, San Bruno, CA, USA) modified for Iranian foods and daily intakes of energy and some nutrients were obtained.
Sample size and intervention
To determine the sample size considering a percent change of 15% in primary outcome of total cholesterol and based on information obtained from a pilot study of size 5 and utilizing the Pocock formula it was estimated to be 24 per group to achieve 95% confidence interval and a power of 80%. Given dropout rate of 30% the sample size increased to 32 in each group.
Participants were randomly assigned to one of the two groups using four-factor Randomized Block Design (RBD in sequentially numbered containers) with allocation ratio 1:1. In each block controls were matched to cases on available data, i.e., age, the place of residence (city or rural) and severity of the disease (according to DAS-28: remission, mild and moderate). Vitamin K1 or placebo pills were given to each subject. Experimental group received a daily chewable pill of 10 mg phylloquinone (manufactured by Minoo Company, Iran) for 8 weeks. The dosage of vitamin K was used in other clinical trials was in various ranges (from microgram to milligram) and it seems there is not enough evidence for optimum doses of vitamin K. Even 45 mg/day of vitamin K2 was recently used in rheumatoid arthritis patients focusing its anti-inflammatory effects [22]. In addition to using different high doses in past researches [22,23,24] and safety of high doses of vitamin K [25], given the only available dosage in Iran, we considered to use 10 mg/day pills of vitamin K1.
Control group received similar amounts of mannitol as the placebo which was specially designed for this study (prepared in Faculty of Pharmacy, Tabriz University of Medical Sciences, Iran). Both of the two supplements had similar taste and appearance and were provided to the volunteers in similar boxes. Participants, caregivers and those assessing the outcomes were blinded to group. Patients in each group were instructed to consume one pill daily after lunch. All participants were asked to return the boxes to the research team at the end of intervention, to calculate compliance of drug for each subject.
The participants received a weekly phone call for monitoring the supplements consumption according to the protocol and being aware of probable side effects of the pills.
Biochemical measurements
Blood samples (7 ml) were collected at baseline and at the end of trial after an overnight fasting. Then, the samples were centrifuged at 3,000 rpm for 15 min (Beckman Avanti J-25; Beckman Coulter, Brea, CA) at room temperature. Then, the serums were frozen in -70℃ until further analysis. However, prothrombin time test (PT test) was immediately conducted on the day of sampling.
Assessment of prothrombin time and lipid profile
In order to screening patients with coagulation abnormalities, prothrombin time was measured for all the participants before the trial. 2-3 cc of blood samples in citrated tubes were centrifuged (at 3,000 rpm for 20 min). Then reagent containing phospholipids (thromboplastin) and CaCl2 (0.025M) added to the plasma tubes. The time required for clot formation is measured by stopwatch (manual method). Time was reported in seconds (prothrombin time). Laboratory reference range value was considered 10-13.5 seconds. Patients who had normal PT or a higher value participated in the study.
Serum levels of total cholesterol (TC), high-density lipoprotein (HDL) and triglyceride (TG) were assayed by enzymatic method (Pars azmoon Kit, Iran). Serum level of low-density lipoprotein (LDL) was determined by Friedewald formula: LDL = TC-HDLTG/5.0 (mg/dL) [26].
Medical Ethical Committee of Tabriz University of Medical Science approved this study and the registration number of this trial in Iranian Registry of Clinical Trials (IRCT) was www.irct.ir/IRCT201205203140N4.
Statistical analysis
All analyses were performed based on Intention To Treat principle. The Statistical SPSS software program (version 11.5; SPSS Inc., Chicago, IL) was used for all the statistical analyses. The data were checked for normality using Kolmogorov-Smirnov test. Presentation of data was as mean (standard deviation), median (percentile 25, percentile 75) and frequency (percentage). Baseline indices were compared in vitamin K group versus placebo group to examine if the randomization was appropriate. Independent sample-t test, Mann-Whitney U test and Fisher exact test were used to compare baseline values between two groups. In order to determination the effects of treatment at the end of intervention, relevant confounders .i.e; duration of RA, energy intake, weight, prothrombin time and baseline values were adjusted (ANCOVA test).
Moreover, in order to assay differences between before and after intervention within groups, Paired sample-t test, Wilcoxon Signed Ranks and Sign test were used [27,28,29]. Statistical significance was set at P < 0.05.
RESULTS
Baseline characteristics
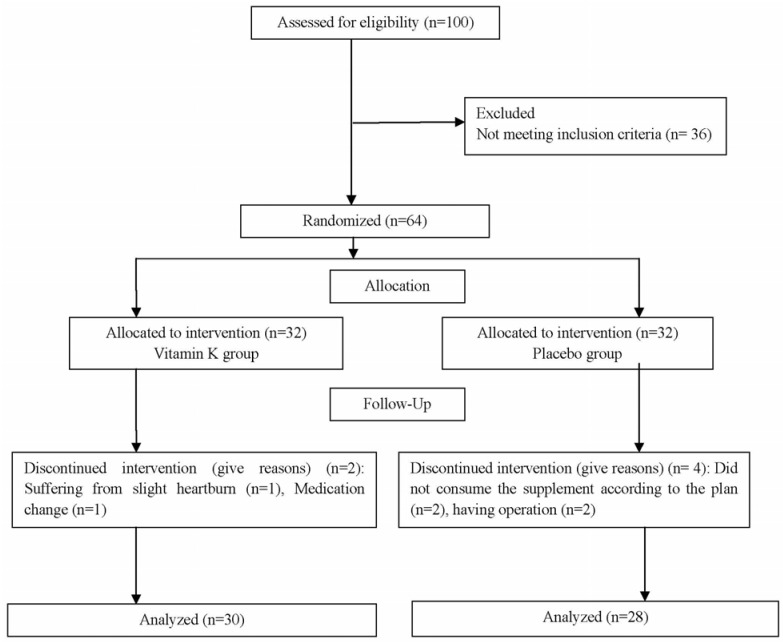
Of sixty four participants assigned to the trial, fifty eight patients completed the eight-week follow up (n = 30 in phylloquinone group and n = 28 in placebo group). No significant side effect in the two study groups was detected. Only one patient who had experienced a slight heartburn at the beginning of intervention was excluded from the study. The diagram of study has been presented in Fig. 1.
Fig. 1. Flow chart of the study.
Baseline characteristics in vitamin K and placebo groups are shown in Table 1. No significant differences were observed in baseline characteristics between two groups with regards to age, BMI, DAS-28, prothrombin time, physical activity and stress level (P > 0.05). The patients' medications were routine drugs in RA treatment maintained as the same drug and dosage throughout the clinical trial period. No one consumed lipid lowering drugs during the study period. Compliance to the supplements provided to participants in this study was calculated through dividing the number of consumed pills by the total number of supplied pills to each subject. The compliance estimated was on average 92% in vitamin K group and 87% in placebo group which wasn't significantly different between two groups (P = 0.206).
Table 1. Characteristics of RA patients at baseline in both groups.
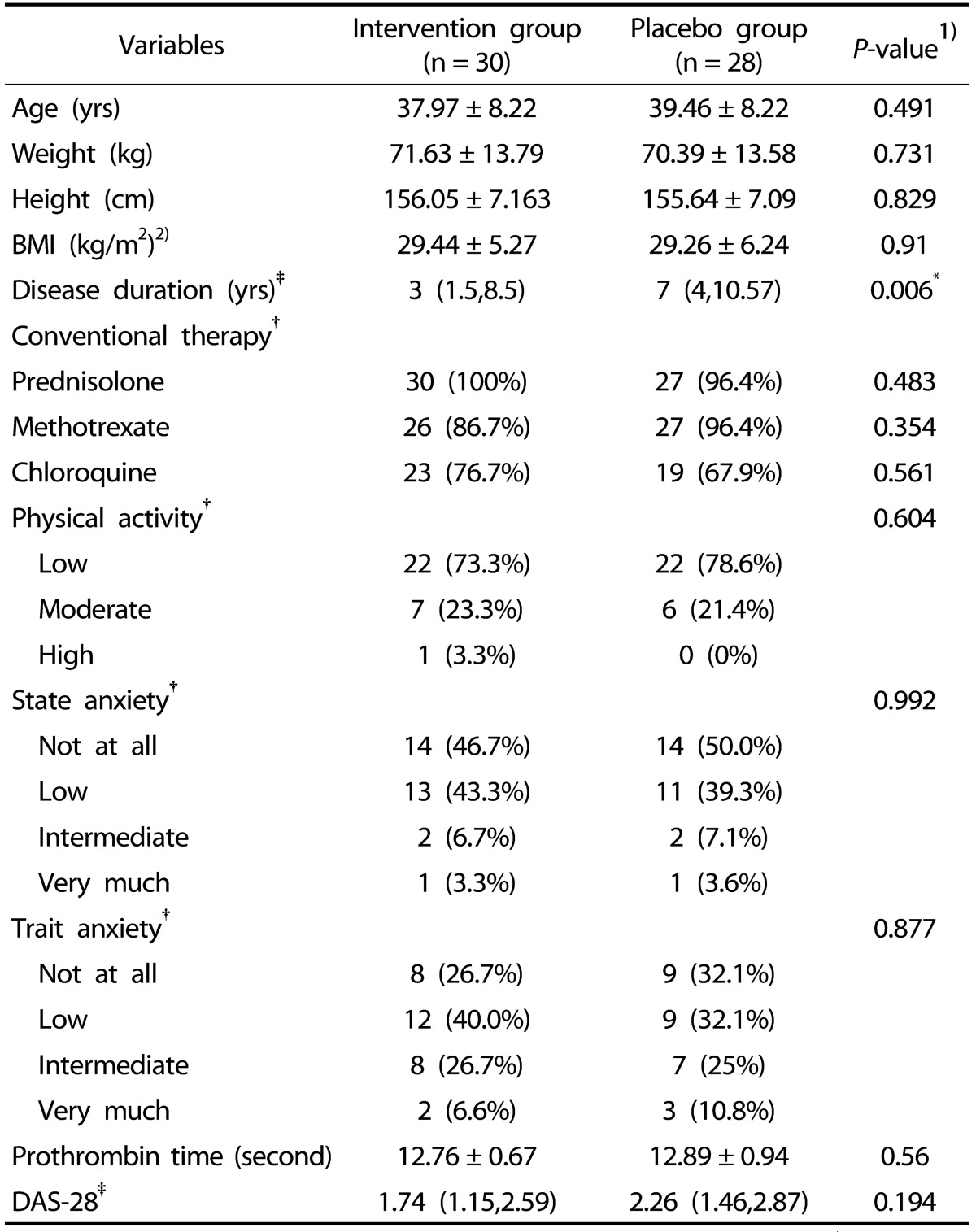
Data are presented as mean ± SD for normally distributed variables, †frequency (percent) for categorical variables and ‡median (percentile 25, percentile 75) for non normally distributed variables.
1)Compare baseline characteristics between two groups (Independent-sample t test for normally distributed variables, Fisher exact tests for categorical variables and Mann-Whitney U test for non normally distributed variables).
2)BMI, body mass index; DAS-28, Disease Activity Score 28.
*Significant difference between groups (P < 0.05)
Dietary intakes before and after intervention period
Regarding Table 2 daily dietary intakes showed no significant differences between two groups before and after intervention (P > 0.05).
Table 2. Daily dietary intakes in women with RA at baseline and after 8 weeks in both groups.
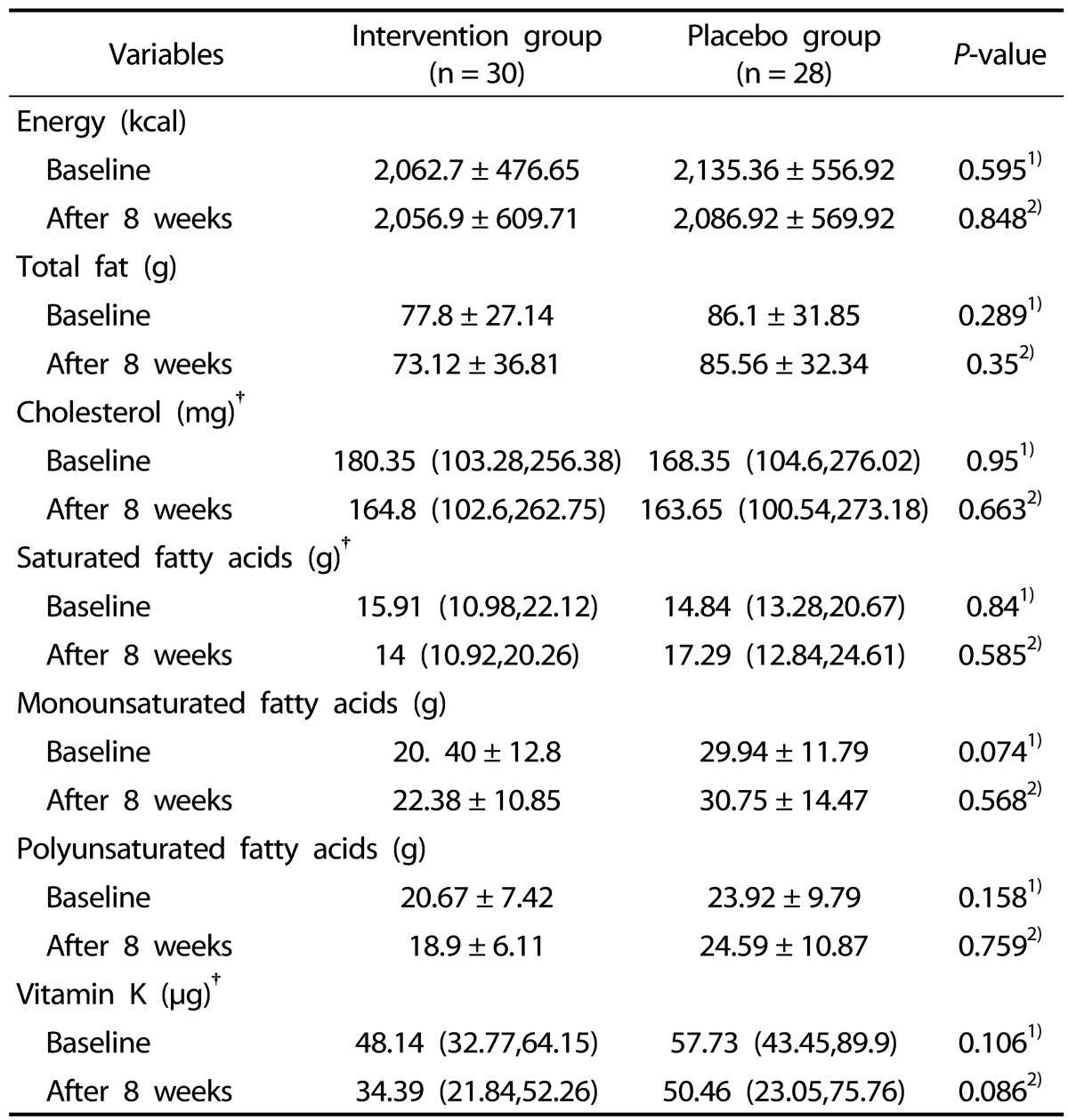
Data are presented as mean ± SD for normally distributed variables, †median (percentile 25, percentile 75) for non normally distributed variables.
1)Compare baseline characteristics between two groups (Mann-Whitney U test for non normally distributed variables and Independent-sample t test for normally distributed variables).
2)Final comparison after intervention period; adjusted for confounders .i.e; duration of RA, energy intake, weight, PT and baseline values (ANCOVA).
Lipid profile before and after intervention period
Serum levels of lipid related markers such as total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglyceride (TG) in both groups before and after intervention are presented in Table 3. Neither at beginning nor at the end of intervention the lipid related markers had significant differences between two groups. Even after adjusting for some confounders such as duration of RA, energy intake, weight, prothrombin time and baseline values, results of analysis of covariance did not show any significant differences in the levels of these markers between two groups at the end of the study (P > 0.05). Although, given the differences (with 95% confidence interval), surprisingly we noticed a worse serum lipid profile at the end of intervention in both groups that wasn't significantly different between before and after intervention values within groups (P > 0.05).
Table 3. Lipid profile and Lipid ratios in women with RA at baseline and after 8 weeks in both groups.
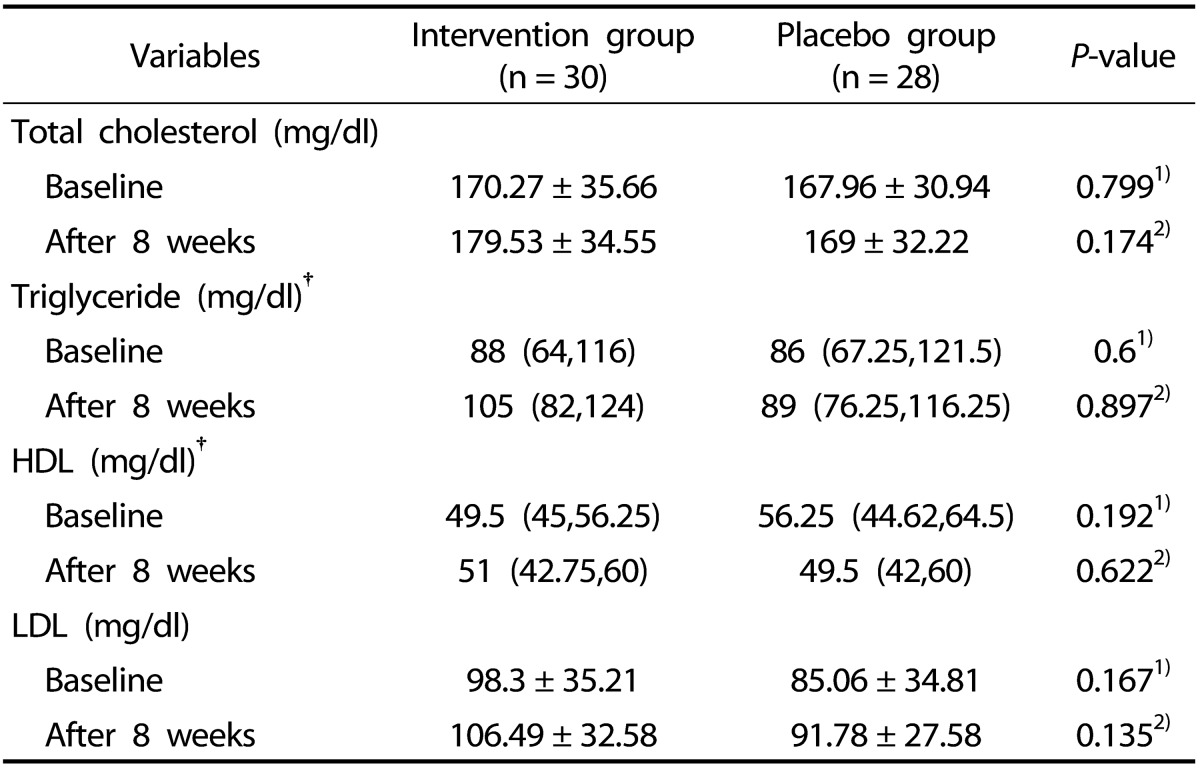
LDL-C, Low Density Lipoprotein; HDL-C, High Density Lipoprotein.
Data are presented as mean ± SD for normally distributed variables, †median (percentile 25, percentile 75) for non normally distributed variables.
1)Baseline vitamin K group vs. baseline placebo group (Independent-sample t test for normally distributed variables and Mann-Whitney U for non normally distributed variables).
2)Final comparison after treatment with vitamin K or placebo; adjusted for confounders .i.e; duration of RA, energy intake, weight, PT and baseline values (ANCOVA).
DISCUSSION
The present study showed that phylloquinone supplementation at dose of 10 mg per day for 8 weeks did not lead to any significant changes in lipids profile in women with rheumatoid arthritis. A few studies investigating the effects of vitamin K on lipid related markers to date reported conflicting results.
The study conducted by Kawashima et al. [12] in hypercholesterolemic rabbits showed that vitamin K2 significantly decreased plasma total cholesterol, lipid peroxidation and ester-cholesterol deposition in the aortas of rabbits. Another experimental study in rats indicated a significant reduction in serum triglyceride levels following vitamin K1 and K2 rich diets (600 mg/kg/day). In their study the reduction in triglycerides level in phylloquinone group was 48% versus 29% in menaquinone group compared with the control. These data support a presumptive favorable effect of vitamin K1 on lipid profile [11]. Human investigations in the literature in this field are scanty. One study which conducted in dialysis patients who had osteoporosis due to secondary hyperparathyroidism, found a similar results to the previous experimental researches. Their study showed that administration of daily 45 mg MK-4, reduced serum total cholesterol significantly, without influencing triglyceride and low-density lipoprotein serum levels [13]. In contrast to aforementioned findings, we observed no significant lipid lowering effect of phylloquinone. Surprisingly, we found a little non significant increase in the levels of blood lipids after intervention period in both groups. In support of our findings, a study by Kristensen et al. [14] has reported that six weeks phylloquinone supplementation in postmenopausal women not only didn't reach an improvement in lipid profile but also it induced an unfavorable effect on lipid profile. Inefficiency of food frequency questionnaire in exact determination of phylloquinone intake, not considering the baseline serum levels of lipid related markers and remarkable dropouts through the study were declared as explanations of these surprising findings [14]. However, a growing body of documents supports a pivotal role of vitamin K in improvement of lipid profile and coronary heart disease, little information is available about the mechanisms of this function. Understanding the mechanisms might explain these discrepant data. In this regard, several cell pathways which vitamin K influences to alter serum lipid related markers are proposed. Some certain proteins which are necessary for vascular tissue health are activated via vitamin K-dependant gamma-carboxylation process. Among these proteins, carboxylated form of matrix Gla-protein (MGP) prevents the infiltration of calcium into the vascular tissues. Thus, calcification of soft tissues may not happen in presence of vitamin K [10].
Likewise, in the study by Takeuchi et al. [30] it's been found that inhibition of adipocytes formation and fat deposition were induced only by vitamin K2 (MK-4) administration in mouse bone marrow cell cultures. Whereas, Sogabe et al. [11] demonstrated significant reductions in weights of total fat and visceral fat in both groups of rats fed by vitamin K1 and K2 rich diets. It has been thought that there are some functional differences between phylloquinone and menaquinones, in results of their structural differences including redox properties or functions of their side chains and also differences in the tissue-specific utilization of the various forms of vitamin K that cause inconsistent results [14].
One possible explanation is that the optimum dose of vitamin K for its beneficial effect on lipid profile has not been defined yet. Thus, the dosage chosen in our trial may not be proper and it could influence phylloquinone's function. A number of investigators suggest that various dosage of vitamin K1 may change its important and useful action in controlling inflammation [31] and consequently in improvement of blood lipids.
In addition, the intervention period may have been insufficient to reach any significant change in lipid profile. Furthermore, consumption of routine drugs in controlling RA results in remission in our study subjects (according to DAS-28 in two groups) which may justify these findings.
There are several strengths and limitations in our study. One of the strengths of present study was the design of study as randomized, double-blinded placebo-controlled trial. Moreover, ideal randomization was conducted in the study and two groups were matched in plenty of variables at the beginning of the intervention such as disease severity, physical activity, anxiety level, etc. This study as a first study assessed dietary intakes, anxiety level and physical activity to control their probable effects on blood lipids through the intervention period. Besides, all the participants had high compliance of drug and given to exclusion criteria subjects with low adherence in each group were excluded from the study.
The limitation of this intervention was confining the trail to a special group of patients with rheumatoid arthritis, and it would be better to repeat this study in a big target population such as involving postmenopausal women with RA, patients in severe stage of the disease (DAS28 > 5.1), and patients with vitamin K deficiency. On the other hand, as aforementioned the probable role of vitamin K might appear obviously at various dosages or after long-term intervention or in larger sample size.
In conclusion, phylloquinone supplementation (10 mg/day) did not alter serum lipid profile in women with RA after eight weeks.
ACKNOWLEDGEMENT
We wish to thank Nutrition Research Center of Tabriz University of Medical Sciences, Tabriz, Iran which supported present research grant; and also we are thankful to women who participated in the study. This article was written based on the data from MS thesis of nutrition registered in Tabriz University of Medical Science under A/138 number.
References
- 1.Abid Mahdi E, Ali Mohamed L, Abass Hadi M. The relationship between lipid profile and inflammatory markers in patients with early rheumatoid arthritis. Iraqi Natl J Chem. 2012;47:391–400. [Google Scholar]
- 2.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD, Merkel PA, Pillemer SR, Reveille JD, Stone JH National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 3.Monjamed Z, Razavian F. The impact of signs and symptoms on the quality of life in patients with rheumatoid arthritis referred to the hospitals of tehran university of medical sciences in year 2005. Qom Univ Med Sci J. 2007;1:27–35. [Google Scholar]
- 4.Nurmohamed MT. Atherogenic lipid profiles and its management in patients with rheumatoid arthritis. Vasc Health Risk Manag. 2007;3:845–852. [PMC free article] [PubMed] [Google Scholar]
- 5.Dessein PH, Christian BF, Solomon A. Which are the determinants of dyslipidemia in rheumatoid arthritis and does socioeconomic status matter in this context? J Rheumatol. 2009;36:1357–1361. doi: 10.3899/jrheum.090288. [DOI] [PubMed] [Google Scholar]
- 6.White D, Fayez S, Doube A. Atherogenic lipid profiles in rheumatoid arthritis. N Z Med J. 2006;119:U2125. [PubMed] [Google Scholar]
- 7.Georgiadis AN, Papavasiliou EC, Lourida ES, Alamanos Y, Kostara C, Tselepis AD, Drosos AA. Atherogenic lipid profile is a feature characteristic of patients with early rheumatoid arthritis: effect of early treatment--a prospective, controlled study. Arthritis Res Ther. 2006;8:R82. doi: 10.1186/ar1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohsaki Y, Shirakawa H, Hiwatashi K, Furukawa Y, Mizutani T, Komai M. Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat. Biosci Biotechnol Biochem. 2006;70:926–932. doi: 10.1271/bbb.70.926. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto H. Vitamin K and rheumatoid arthritis. IUBMB Life. 2008;60:355–361. doi: 10.1002/iub.41. [DOI] [PubMed] [Google Scholar]
- 10.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr. 2004;134:3100–3105. doi: 10.1093/jn/134.11.3100. [DOI] [PubMed] [Google Scholar]
- 11.Sogabe N, Maruyama R, Baba O, Hosoi T, Goseki-Sone M. Effects of long-term vitamin K(1) (phylloquinone) or vitamin K(2) (menaquinone-4) supplementation on body composition and serum parameters in rats. Bone. 2011;48:1036–1042. doi: 10.1016/j.bone.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Kawashima H, Nakajima Y, Matubara Y, Nakanowatari J, Fukuta T, Mizuno S, Takahashi S, Tajima T, Nakamura T. Effects of vitamin K2 (menatetrenone) on atherosclerosis and blood coagulation in hypercholesterolemic rabbits. Jpn J Pharmacol. 1997;75:135–143. doi: 10.1254/jjp.75.135. [DOI] [PubMed] [Google Scholar]
- 13.Nagasawa Y, Fujii M, Kajimoto Y, Imai E, Hori M. Vitamin K2 and serum cholesterol in patients on continuous ambulatory peritoneal dialysis. Lancet. 1998;351:724. doi: 10.1016/S0140-6736(05)78492-0. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen M, Kudsk J, Bügel S. Six weeks phylloquinone supplementation produces undesirable effects on blood lipids with no changes in inflammatory and fibrinolytic markers in postmenopausal women. Eur J Nutr. 2008;47:375–379. doi: 10.1007/s00394-008-0737-4. [DOI] [PubMed] [Google Scholar]
- 15.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 16.Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S467–S472. doi: 10.1002/acr.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9:755–762. doi: 10.1079/phn2005898. [DOI] [PubMed] [Google Scholar]
- 18.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 19.Hazavehei SM, Asadi Z, Hassanzadeh A, Shekarchizadeh P. Comparing the effect of two methods of presenting physical education II course on the attitudes and practices of female students towards regular physical activity in Isfahan University of Medical Sciences. Iran J Med Edu. 2008;8:121–131. [Google Scholar]
- 20.Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, Aletaha D, van Riel PL. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68:954–960. doi: 10.1136/ard.2007.084459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghaffarpour M, Houshyar Rad A, Kianfar H. Guideline for Household Measures, Conversion Coefficients and the Percent of Edible Food. Tehran: Publication of Agricultural Sciences; 2000. [Google Scholar]
- 22.Ebina K, Shi K, Hirao M, Kaneshiro S, Morimoto T, Koizumi K, Yoshikawa H, Hashimoto J. Vitamin K2 administration is associated with decreased disease activity in patients with rheumatoid arthritis. Mod Rheumatol. 2013;23:1001–1007. doi: 10.1007/s10165-012-0789-4. [DOI] [PubMed] [Google Scholar]
- 23.Braam LA, Knapen MH, Geusens P, Brouns F, Vermeer C. Factors affecting bone loss in female endurance athletes: a two-year followup study. Am J Sports Med. 2003;31:889–895. doi: 10.1177/03635465030310062601. [DOI] [PubMed] [Google Scholar]
- 24.Craciun AM, Wolf J, Knapen MH, Brouns F, Vermeer C. Improved bone metabolism in female elite athletes after vitamin K supplementation. Int J Sports Med. 1998;19:479–484. doi: 10.1055/s-2007-971948. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher ML. Intake: the nutrients and their metabolism. In: Mahan LK, Escott-Stump S, Raymond JL, editors. Krause's Food & the Nutrition Care Process. 13th ed. St. Louis (MO): Elsevier/Saunders; 2012. pp. 80–81. [Google Scholar]
- 26.Cordova CM, Schneider CR, Juttel ID, Cordova MM. Comparison of LDL-cholesterol direct measurement with the estimate using the Friedewald formula in a sample of 10,664 patients. Arq Bras Cardiol. 2004;83:482–487. 76–81. doi: 10.1590/s0066-782x2004001800006. [DOI] [PubMed] [Google Scholar]
- 27.Asghari Jafarabadi M, Mohammadi SM. Statistical series: summarizing and displaying data. Iran J Diabetes Lipid Disord. 2013;12:83–100. [Google Scholar]
- 28.Asghari Jafarabadi M, Mohammadi SM. Statistical series: introduction to statistical inference (Point estimation, confidence interval and hypothesis testing) Iran J Diabetes Lipid Disord. 2013;12:173–192. [Google Scholar]
- 29.Asghari Jafarabadi M, Soltani A, Mohammadi SM. Statistical series: tests for comparing of means. Iran J Diabetes Lipid Disord. 2013;12:265–291. [Google Scholar]
- 30.Takeuchi Y, Suzawa M, Fukumoto S, Fujita T. Vitamin K(2) inhibits adipogenesis, osteoclastogenesis, and ODF/RANK ligand expression in murine bone marrow cell cultures. Bone. 2000;27:769–776. doi: 10.1016/s8756-3282(00)00396-3. [DOI] [PubMed] [Google Scholar]
- 31.Shea MK, Dallal GE, Dawson-Hughes B, Ordovas JM, O'Donnell CJ, Gundberg CM, Peterson JW, Booth SL. Vitamin K, circulating cytokines, and bone mineral density in older men and women. Am J Clin Nutr. 2008;88:356–363. doi: 10.1093/ajcn/88.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]