Abstract
Background
There is increasing evidence that the dorso-lateral prefrontal cortex (DLPFC), a brain region related to reward and motivational processes, is involved in effective response inhibition and that decreased activity in this region coincides with reduced inhibitory capacity. Using transcranial direct current stimulation (tDCS) to manipulate cortical activation, this study examined whether cross-hemispheric tDCS over the DLPFC affected performance on an inhibitory control task.
Methods
Neurologically intact participants performed a modified Stroop color-word matching task before and after completing one of two tDCS conditions; (1) anodal stimulation over the left DLPFC or (2) sham tDCS.
Results
There was a statistically significant effect of tDCS condition on Stroop reaction time (RT) pre-post tDCS change scores. Participants who received anodal stimulation over the left DLPFC demonstrated statistically significant faster RT change scores on the Stroop items compared to participants in the sham condition. Although errors on Stroop incongruent items decreased before and after receiving the tDCS treatment, there were no significant differences in errors on Stroop items between the anodal stimulation over left DLPFC and sham tDCS conditions. Anodal tDCS, which is known to elevate neural excitation, may have enhanced activation levels in the left DLPFC and minimized impairment of inhibitory control, resulting in better task performance.
Conclusions
Current findings provide preliminary evidence that increased excitation of the left DLPFC improves inhibitory control and are a step toward understanding the potential of tDCS for moderating deficits in inhibitory control.
Keywords: Dorsolateral prefrontal cortex, executive functioning, self-control, stroop, transcranial direct current stimulation
Introduction
Recent research indicates that inhibitory control, or response inhibition, is a key feature of self-control and may impact upon an individual's ability to inhibit impulsive responses to stimuli (Friese et al. 2008; Hofmann et al. 2009; Fujita 2011). For example, a compromised ability to inhibit impulsive responses is associated with increased consumption of high calorie food (Guerrieri et al. 2012), higher alcohol intake (Houben et al. 2011), and a propensity toward obesity (Guerrieri et al. 2008). Furthermore, research has demonstrated that impaired inhibitory control is significantly related to many behaviors that require impulse suppression such as food consumption (Hagger et al. 2013b), smoking urges (Hagger et al. 2013a), and alcohol-seeking behavior (Muraven and Shmueli 2006).
A number of studies suggest that modulating an individual's inhibitory control for alcohol and food-related cues impacts upon their subsequent consumption of alcohol and palatable foods in ostensible taste-and-rate tasks (Houben et al. 2011; Jones et al. 2011). For example, Houben et al. (2011) examined whether increasing or decreasing inhibitory control impacted food intake. Participants completed an initial response inhibition task, the stop-signal task (SST; Logan et al. 1997). Following this, participants completed either (1) an inhibition condition in which one type of food was always paired with a stop signal, or (2) an impulsive condition in which another type of food was never paired with a stop signal. The consistent mapping of one type of food onto stop signals was purported to increase inhibitory control capacity for that food type. Conversely, the type of food which was never paired with a stop signal (impulsive condition) would lead to decreased ability to inhibit responses to those stimuli and increase impulsivity in response to that food. Participants also completed a bogus taste test of the foods presented in the SST, during which calorific consumption was monitored. Increasing inhibition toward a particular food decreased the subsequent consumption of that food in the taste-test phase of the study, whereas decreasing inhibition (increasing impulsivity) toward a particular food increased intake of that food.
In a similar study, Jones et al. (2011) examined the impact of priming inhibitory control using an SST paradigm on alcohol-seeking behavior. Participants were randomly assigned to SST groups that differed in terms of the emphasis placed upon the importance of successful inhibition. Participants in the ‘disinhibition’ group were informed that rapid responding was the most important task, whereas participants in the ‘restraint’ group were informed that successful inhibition was to be prioritized. Participants were then asked to rate the pleasantness of different drinks, including a beer they believed to contain alcohol. The results indicated that participants in the ‘disinhibition’ group, who had been informed to prioritize response speed, consumed more beer than participants in the ‘restraint’ group who were instructed to prioritise inhibition. These findings led Jones et al. to suggest that a temporary loss of inhibitory control impacts upon motivated behavior such as alcohol-seeking.
These studies provide evidence that ‘strengthening’ or ‘training’ inhibitory control through repeated ‘practice’ on response inhibition tasks impacts upon behavior in situations requiring self-control, potentially by increasing resistance to temptation and impulsive cue-driven responding. These findings have been replicated elsewhere (e.g., Veling et al. 2011, 2013a,b; Todd and Mullan 2013). The underlying neural mechanism for such strengthening of inhibitory control, however, remains unclear. A number of studies implicate dorso-lateral prefrontal cortex (DLPFC) activation during tasks and behaviors involving response inhibition and self-control (MacDonald et al. 2000; Knoch et al. 2006; Glascher et al. 2009; Hare et al. 2009; Figner et al. 2010; Heatherton and Wagner 2011; Friese et al. 2013). For example, Steinbeis et al. (2012) examined children's decision-making abilities while playing two different games, only one of which required participants to exert self-control. Functional magnetic resonance imaging (fMRI) results revealed left DLPFC activation only when participants played the game which required them to exert self-control. Similarly, Friese et al. (2013) demonstrated that engaging in an emotion suppression task requiring individuals to actively inhibit their responses to emotionally evocative stimuli coincided with reduced performance on a subsequent Stoop color-word matching task, a task that has frequently been implicated in the literature as a measure of response inhibition and the inhibition component of self-control.1 Importantly, the decrement in performance coincided with reduced activity in the DLPFC verified by fMRI. Deficits in this brain region, shown to be correlated with reward and motivational processes, appear to be implicated in effective response inhibition and decreased activity in this region coincides with reduced capacity for response inhibition (Heatherton 2011; Hedgcock et al. 2012; Friese et al. 2013; Hagger and Chatzisarantis 2013). Given this evidence, we propose that increased activity in the DLPFC may enhance inhibitory control and, therefore, performance on tasks requiring self-control. In this study, we stimulated DLPFC activity using transcranial direct current stimulation (tDCS) and examined subsequent effects on performance on an inhibitory control task.
tDCS is a noninvasive method of brain stimulation that can be used to modulate cortical excitability. When applied to the skull, tDCS penetrates the underlying cortex and increases (anodal) or decreases (cathodal) cortical excitability in that area (Nitsche and Paulus 2000; Zaghi et al. 2010; Lang et al. 2011). tDCS can be applied in a cross-hemispheric manner, whereby the anodal electrode is applied to one hemisphere and the cathodal to the other. Recent research suggests that cross-hemispheric tDCS over DLPFC improves performance on numerous tasks associated with executive functioning including task-switching tasks (Leite et al. 2012) and working memory (Jeon and Han 2012), and as well as modifying impulsive responses such as amelioration of risk-taking behaviors (Fecteau et al. 2007; Boggio et al. 2010). For example, Fecteau et al. (2007) examined the impact of cross-hemispheric tDCS over DLPFC on the Balloon Analogue Risk Task (BART), a behavioral analog of risk-taking and impulsivity that correlates well with ‘real world’ measures of risk-related behaviors (Lejuez et al. 2002). Participants received either: (1) anodal over the right and cathodal over the left DLPFC (ARCL), (2) anodal over the left and cathodal over the right DLPFC (ALCR), or (3) sham stimulation during the risk task. Participants in the ARCL group demonstrated less risk taking on the BART compared to those in sham or ALCR tDCS groups. This led Fecteau et al. (2007) to suggest that the interhemispheric balance of activation across the DLPFC cortices contributes to decision-making behavior, and that altering this balance impacts upon risk taking.
This study examined the impact of cross-hemispheric tDCS over the DLPFC on inhibitory control using a Stroop color-word matching task in neurologically intact participants. The techniques associated with tDCS are a developing science and precise predictions are somewhat difficult to make, since effective dose and duration for a given task remain unclear (Jacobson et al. 2012). Based on the recent finding that left DLPFC is primarily activated during response inhibition and self-control tasks, we predicted that participants receiving an anodal tDCS over the left DLPFC, the configuration linked with increased activity in the DLPFC and performance on tasks correlated with processing in this region, would be more likely to improve inhibitory control than participants receiving sham tDCS. Specifically, we predict that participants will demonstrate better Stroop-task performance, as indicated by faster averaged response latency and decreased error rates, after receiving anodal tDCS over the left DLPFC and controlling for baseline, relative to Stroop performance of participants receiving the sham tDCS.
Method
Participants
Twenty-eight neurologically intact, right-handed (in accord with the Edinburgh Handedness Inventory; Oldfield 1971) undergraduate students took part in the study (18 females, mean age 24.5 years). All participants had normal or corrected to normal vision. The study was approved by the Curtin University Human Research Ethics Committee. All participants provided written informed consent.
Procedure and materials
Participants were randomly assigned to one of two tDCS conditions; (1) anodal stimulation of left dorso-lateral prefrontal cortex (DLPFC) or (2) sham (no tDCS). Participants were naive to the tDCS condition to which they were assigned. Within each session, participants completed the following tasks in this order: (1) pre-tDCS Stroop task, (2) tDCS, and (3) post-tDCS Stroop task. Fourteen participants were assigned to each tDCS condition.
Stroop task
A computerized version of a modified Stroop color-word matching task was used to measure inhibitory control. Following the task instructions, two strings of letters, presented in 140 point font size, were simultaneously presented on a LCD computer screen, one at the top and one at the bottom, 100 pixels either side of the midpoint of the screen. The string of letters at the top of the screen was always presented in one of four colors (yellow, red, blue, or green). These letters were either nonwords (i.e., a set of randomly scrambled letters) or spelled a color word (i.e., yellow, red, blue, or green). The string of letters at the bottom of the screen was always presented in gray color. Participants were instructed to decide, as quickly and accurately as possible, whether the color of the letter string at the top of the screen matched the meaning (name of color) of the string at the bottom. Participants responded by clicking the left mouse button for a ‘match’ response and the right mouse button for a ‘non-match’ response. Participants first completed seven practice trials, followed by 64 test trials (32 neutral and 32 incongruent). In the neutral trials, the target word was a random string of letters (e.g., “NSGL”) in which the color of the string presented at the top of the screen matched the meaning of the string presented at the bottom of the screen. In incongruent trials, the letter string presented at the top of the screen was a real word that differed in both color and meaning (e.g., the word “BLUE” in the color red) to the string presented at the bottom of the screen. Trials were counterbalanced and randomly presented. Stimuli remained on the screen until a response was given, or until 5 sec had passed. A blank screen was then briefly presented for 1000 ms. The presentation of a fixation cross of size 100 × 100 pixels in the center of the screen indicated the start of a new trial. The task took approximately 10 min to complete. Reaction time (RT) to each stimulus item was recorded in millisecond (ms) from the onset of the stimulus presentation until the response was detected of 5 sec had passed. Errors were recorded as the number of incorrect trials.2
Transcranial direct current stimulation (tDCS)
Immediately following the pre-tDCS Stroop task, each participant commenced the tDCS phase of the study. tDCS was delivered by a battery-driven, constant current stimulator (Soterix™ 1x1). A constant current of 2 mA was applied for 10 min with a pair of 35 cm2 sponge electrodes soaked in saline solution (equivalent to 0.057 mA/cm2). There was a ramp up/ramp down period of 30 sec at the start and end of tDCS. Participants received either anodal stimulation over the left DLPFC or sham tDCS. Anodal stimulation over the left DLPFC was performed with the anode placed over F3 (using the 10–20 system) and the cathode placed over the right DLPFC (F4 using the 10–20 system). Sham stimulation was conducted with the same montage, with 30 sec of tDCS applied at onset, after which the current stimulator was de-ramped. tDCS was administered for 10 min, during which time the participant watched a short video (comedy sketch, all participants watched same video). Following administration of tDCS, participants completed the post-tDCS Stroop task, which was the same as the pre-tDCS Stroop task except for the order of trials. Upon completion of tDCS, all participants were asked if they could tell whether they received stimulation or not (yes or no response).
Results
All participants successfully completed the experiment and there were no missing data. No participant reported any adverse effects of tDCS. Data from all 28 participants were used in analysis.
The stroop effect
Bonferroni-adjusted paired-samples t-tests revealed statistically significant differences between neutral and incongruent Stroop stimuli words pre-tDCS for reaction time (RT), t27 = −8.14, P < 0.001, d = 1.11, and error rates, t27 = −4.61, P < 0.001, d = 0.97. Participants responded faster (M = 957.82, SD = 147.69) and exhibited fewer errors (M = 1.14, SD = 1.60) on neutral trials compared to incongruent trials (RT: M = 1150.37 SD = 198.61; Errors: M = 3.36, SD = 2.25). Analogously, participants also exhibited statistically significant faster RTs (M = 815.46, SD = 185.33; t27 = −5.35, P < 0.001, d = 0.79) with fewer errors (M = 0.50, SD = 0.79; t27 = −3.39 P = 0.002, d = 0.89) on post-tDCS neutral trials compared to incongruent trials (RT: M = 990.44, SD = 250.68; Errors: M = 1.60, SD = 1.57). These findings are consistent with the Stroop effect in that participants are expected to respond more quickly and accurately on neutral trials compared to incongruent trials. Mean actual RTs and error scores for each trial type and tDCS condition are presented for both times in Figures1A and B.
Figure 1.
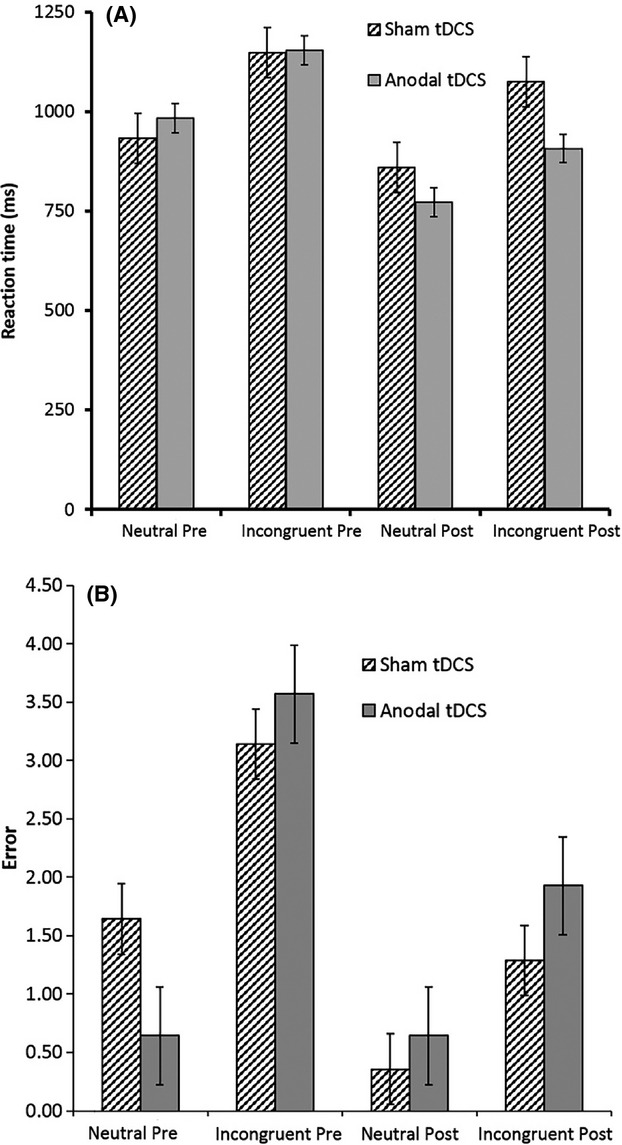
(A) Mean reaction times for each trial type (neutral, incongruent) at each time (pre-tDCS, post-tDCS), including standard error. (B) Mean error scores for each trial type (neutral, incongruent) at each time (pre-tDCS, post-tDCS), including standard error.
Reaction time
Reaction time scores were entered into analysis as raw scores. To examine the impact of tDCS group on neutral and incongruent trials of the Stroop task, a repeated measures analysis of variance (ANOVA) with tDCS condition (anodal, sham) as the between-groups factor and trial type (neutral, incongruent) and time (pre-tDCS, post-tDCS) as within-groups factors was used. There was no statistically significant main effect of tDCS condition, F1,26 = 0.591, P > 0.05, partial η2 = 0.02. There were statistically significant main effects of time, F1,26 = 38.16, P < 0.001, partial η2 = 0.60, and trial type, F1,26 = 51.75, P < 0.001, partial η2 = 0.66. There was a statistically significant tDCS condition x time interaction effect, F1,26 = 10.08, P < 0.05, partial η2 = 0.25. There were no other statistically significant interaction effects.
To facilitate analysis of the tDCS condition × time interaction effect, RT change scores were calculated by subtracting the mean pre-tDCS RT from the mean post-tDCS RT for each participant, with negative RT change scores indicative of reduced pre-post RT (i.e., the participant was faster) and positive RT change scores indicative of increased pre-post RT (i.e., the participant was slower). Adjusted independent samples t-tests revealed a statistically significant difference between sham and ALCR tDCS conditions on RT change scores for neutral trials, t28 = −3.07, P < 0.05, d = 1.16, and incongruent trials, t28 = −2.74, P < 0.05, d = 1.04. For neutral trials, those who received anodal tDCS demonstrated greater reduction in RT (M = −210.70, SD = 107.64) compared to those in the sham condition (M = −74.03, SD = 127.47). Similarly, for incongruent trials those who received anodal tDCS demonstrated greater reduction in RT (M = −246.91, SD = 158.87) compared to those in the sham condition (M = −72.94, SD = 176.34).3 See Figure1A for all mean RTs.
Errors
Error scores were entered into the analysis as raw scores. To examine the impact of tDCS group on error scores on neutral and incongruent trials, an repeated measures ANOVA with tDCS condition (anodal, sham) as the between-groups factor and time (pre-tDCS, post-tDCS) and trial type (neutral, incongruent) as within-groups factors was conducted. There was no statistically significant main effect of tDCS condition on error scores, F1,26 = 0.05, P > 0.001, partial η2 = 0.002. There was a statistically significant main effect of time, F1,26 = 12.05, P < 0.001, partial η2 = 0.32, and trial type, F1,26 = 27.52, P < 0.001, partial η2 = 0.51, on error scores. There was also a statistically significant time × trial type interaction effect, F1,26 = 4.73, P < 0.05, partial η2 = 0.15. Bonferroni-adjusted pairwise comparisons of error scores revealed a statistically significant difference between pre-tDCS and post-tDCS error scores for incongruent trials, t27 = 3.46, P < 0.05, d = 0.83, but no statistically significant difference between pre-tDCS and post-tDCS error scores for neutral trials, t27 = 1.90, P = 0.68, d = 0.51. Regardless of tDCS condition (anodal, sham), participants exhibited improved accuracy (decreased error) for incongruent trials only. Participants demonstrated significantly reduced error scores from pre-tDCS (M = 3.36, SD = 2.57) to post-tDCS (M = 1.61, SD = 1.57) for incongruent trials.
Discussion
This study examined the impact of anodal tDCS over the left dorso-lateral prefrontal cortex (DLPFC) on inhibitory control as indicated by performance on a modified Stroop color-word matching task. We predicted that anodal tDCS over the left DLPFC would lead to enhanced performance on the Stroop task relative to sham tDCS. Results revealed that mean Stroop reaction times (RTs) for both neutral and incongruent items were statistically significantly reduced, compared to sham, following anodal stimulation over the left DLPFC. Error rates also decreased although there was no statistically significant effect for tDCS condition. This indicates that the reduced RT did not lead to an increase in errors (i.e., a speed–accuracy trade-off). Participants in the anodal tDCS condition had statistically significant reductions in RT and error rates simultaneously relative to the sham condition. Overall, findings provide initial evidence that excitation of the left DLPFC and inhibition of the right DLPFC leads to improvements on an inhibitory control task.
Current findings have important ramifications for the increasing evidence linking inhibitory control to regulation of behavior and adaptive outcomes (e.g., Guerrieri et al. 2008; Hagger et al. 2013a; Guerrieri et al. 2012 #6429; Houben et al. 2011). Our methods were specifically designed to isolate effects of stimulating regions of the brain, specifically, the DLPFC, that have been linked to effective inhibitory control, on response inhibition (Vanderhasselt et al. 2006; Figner et al. 2010; Allom et al. 2015). The Stroop task is acknowledged as a key paradigm to assess response inhibition and, as response inhibition is a fundamental component of the self-control construct (Hofmann et al. 2012), we speculate that current results may have wider implications for regulatory behaviors which require good inhibitory control. For example, research has indicated that reduced inhibitory control is closely associated with behaviors contingent with poorer self-control and reduced behavioral regulation, such as eating and alcohol consumption while, in contrast, better response inhibition capacity is associated with effective self-control and adaptive outcomes (Muraven and Shmueli 2006; Guerrieri et al. 2008, 2012; Houben et al. 2011; Hagger et al. 2013a,b). Furthermore, self-control theorists (Heatherton 2011; Heatherton and Wagner 2011; Harvey 2012; Hagger and Chatzisarantis 2013; Kurzban et al. 2013) and researchers examining individuals performance on response inhibition tasks using imaging techniques (Hedgcock et al. 2012; Friese et al. 2013) have implicated reduced activity in the DLPFC, the region of the brain correlated with motivation and executive functioning, as the mechanism underpinning inhibition failures. This study adds to this body of research by demonstrating that stimulating the same region leads to improved response inhibition on the Stroop task, a task that has been shown to place considerable demand on response inhibition capacity (Hofmann et al. 2012). Current evidence not only provides some corroboration of imaging data but also indicates that stimulating the region leads to adaptive changes in performance on a response inhibition task.
The present findings are also important as they may help to augment the accumulating evidence of research demonstrating that inhibitory control can be improved through engaging in tasks that stimulate response inhibition (Houben et al. 2011; Jones et al. 2011; Rebar et al. 2015; Veling et al. 2011, 2013a,b; Todd and Mullan 2013). These studies demonstrate that engaging in tasks that stimulate inhibitory control over a period of time leads to better behavioral regulation. Researchers surmise that the improvements are due to stimulation of activity in the DLPFC. Our results provide an analog to these findings by demonstrating that stimulating the same region leads to identical effects on response inhibition capacity. The current findings may contribute to the converging evidence for the mechanisms that underpin inhibitory control and an indication of how it can be modulated.
The present findings may have implications for behavioral domains in which lapses in inhibitory control contribute to maladaptive behavioral patterns and associated outcomes. For example, there is evidence that inhibitory control is implicated in eating behavior and conditions associated with overeating such as overweight and obesity (Vohs and Heatherton 2000; Nederkoorn et al. 2010; Hagger et al. 2013b). Reduced left DLPFC activation is associated with both decreased inhibitory control and higher levels of impulsivity in obese populations (Brooks et al. 2013). The finding that increasing activation of left DLPFC improves inhibitory control is therefore consistent with the neurobiological findings concerning brain activation in obesity research, and lends some support to tDCS as a therapeutic intervention in the management of impulsive disorders. Although this study cannot delineate the exact processes underlying improved inhibitory control following excitation of the left DLPFC it could be suggested that anodal tDCS increases synaptic strength and amplifies neural communication in the left DLPFC (Lüscher and Malenka 2012). Alternatively, improved inhibitory control may be attributable to changes in the interhemispheric balance of activation across the DLPFC. The tDCS montage used in this study involved excitation of the left DLPFC and inhibition of the right DLPFC, which would lead to asymmetric interhemispheric activation. It cannot therefore be concluded that increased activation of left DLPFC alone led to improved inhibitory control. Fecteau et al. (2007) suggested that altering the interhemispheric balance of DLPFC activation impacts upon risk taking, which may be the case in this study.
The present findings are analogous to improvements in executive functioning, specifically planning ability following activation of the left DLPFC (Dockery et al. 2009; Leite et al. 2012). As for tasks invoking planning skills, only increased activation of the left DLPFC has been associated with improved inhibitory control in this study. Direct current stimulation of the DLPFC has been shown to reduce alcohol cravings, a behavior thought to be indicative of resilience. For example, researchers have reported that the contralateral application of tDCS decreased cravings for people with alcohol dependence (Boggio et al. 2008) and less risk-taking behavior in marijuana users (Boggio et al. 2010), regardless of the left-right configuration of the anodal-cathodal electrodes over the DLPFC. Similarly, Fregni et al. (2008) reported that smokers experienced a reduced number of nicotine cravings after cross-hemispheric tDCS over both the left and right DLPFC. Boggio et al. (2008) suggested that anodal stimulation or cathodal inhibition of the right or left DLPFC disrupted the balance between the left and right DLPFC activation, such that cravings were reduced. The present findings, however, suggest that increased excitation of the left DLPFC specifically improves inhibitory control. It may be suggested that the pattern of DLPFC involvement depends upon the nature of the self-control ‘task’. There is some evidence to suggest that neuronal activation is much more bilaterally distributed across the DLPFC during craving states (Wilson et al. 2004). We therefore suggest that the left DLPFC is primarily activated when response inhibition, or more basic inhibitory control, is required.
Some limitations of this study should be considered. The most important limitation is that we cannot determine whether improved inhibitory control resulted from increased excitation of left DLPFC, or from changing the balance of activity across both DLPFC cortices. In addition, we cannot confirm that tDCS did not impact on other densely connected areas of prefrontal cortex, such as the ventromedial prefrontal cortex, an area that has also been implicated in self-control (Hare et al. 2009). In addition, we did not collect data examining the effect of TDCS conditions on responses to a simple choice-time reaction task. Comparison of such data with current findings would have tested whether the effects of stimulation of the DLPFC were confined to tasks tapping response inhibition rather than a generalized enhancement of cognitive functioning as the improvements in the neutral Stroop items found in the current study might imply. Collection of data on tasks tapping generalized reaction time as a comparison condition would be necessary to draw unequivocal conclusions as to the nature of the effect. Finally, we did not assess a behavioral manifestation of self-control beyond that of response inhibition using the Stroop. It would be informative to examine the impact of changes in response inhibition on other behaviors requiring or invoking inhibitory control such as caloric intake or alcohol consumption.
This study demonstrates improvements in inhibitory control in people receiving tDCS and is a step toward understanding the neural underpinnings of self-control. The implication is that tDCS may be a valuable strategy for the prevention or treatment of problems relating to self-control. Deficits in response inhibition have also been demonstrated to contribute to clinically-diagnosed impulse disorders, such as obsessive compulsive disorder and attention deficit hyperactivity disorder (Chamberlain and Sahakian 2007). The potential of tDCS as a therapeutic tool for such disorders also remains unexplored. Future tDCS studies should investigate the optimum dose, duration, and electrode configuration required to strengthen the self-control process in both nonclinical groups and those with clinically diagnosed impulse disorders.
Acknowledgments
We acknowledge the support of an Australian Research Council Discovery Project #DP130103277 awarded to M. S. Hagger and E. J. Vanman. We would also like to thank all participants for their contribution to this study.
Footnotes
It is important to note that incongruent or ‘conflict’ versions of the Stroop task, in which individuals are presented with Stroop items that create interference between color-naming and word-reading processes and forcing a suppression of the prepotent response to read the word, taps response inhibition. The Stroop task has been widely acknowledged as one of the predominant paradigms to evaluate response inhibition (Logan et al. 1997; Hofmann et al. 2012) and it has given rise to other tasks that tap the same inhibition processes (Bush et al. 2003). Furthermore, authors have consistently linked inhibitory control, self-control resource depletion, and Stroop task performance (Hagger et al. 2010; Hofmann et al. 2012). Although response inhibition appears to be the focal process underpinning Stroop performance, it is important to recognize that the task is also implicated in other aspects of cognitive processing and executive functioning including selective attention, detection interference, task switching, and cognitive flexibility (Roberts and Hall 2008; Hyafil et al. 2009; Xiao et al. 2009; Coderre and van Heuven 2013).
The version of the Stroop task used in the current experiment is a variant of the single-item computerized version (MacLeod et al. 2005) and taps equivalent word-reading and color-naming processes and the associated interference. The version adopts a protocol first proposed by Dyer (1973) to test the effect of separating the reading and color processes in the presentation of the stimuli on the Stroop effect rather than versions where the reading and color-naming processes in the stimuli are integrated (words appearing in different colored ink), which are more ‘traditional’, as noted by MacLeod (1991; MacLeod et al. 2005). So comparing reaction times to neutral stimuli (e.g., where the string of letters appearing at the bottom of the screen in gray reads “BLUE”, and the string of letters appearing at the top reads “NGSL” and appears in the color blue) with reaction times for incongruent stimuli (e.g., string of letters appearing at the bottom of the screen in gray reads “BLUE”, and the string of letters appearing at the top is a color word “RED”, and appears in the color blue). Dyer tested a number of variants of these conditions to examine the effect of separation of reading and color-naming processes, and his data showed that the Stroop effect tended to be slightly weaker in the separate conditions compared to the integrated conditions, but the effect was still clear. Of relevance to the current study, Dyer's data clearly demonstrated the Stroop effect for sets of stimuli equivalent to those used in the current version of the Stroop task. Specifically, he found that reaction times for the congruent stimuli (termed “incongruent different A”) were significantly slower than the neutral stimuli (termed “control same”), consistent with the Stroop effect. Based on this developmental work with the Stroop task, we are confident that the ‘separated’ version used in the current research taps equivalent processes to versions in which the stimuli are integrated.
For completion, we also conducted these analyses without using change scores and using pre-tDCS Stroop RT scores as covariates. Specifically, in this alternative set of analyses, we compared the effect of tDCS condition (anodal, sham) on post-tDCS Stroop RT scores for neutral and incongruent trials separately while controlling for pre-tDCS scores from both trial types. A one-way ANCOVA with post-tDCS Stroop RT for neutral trials as the dependent variable, tDCS condition as the independent variable, and pre-tDCS Stroop RT scores for neutral and incongruent trials as covariates revealed that participants receiving ALCR tDCS exhibited statistically significantly faster Stroop RT scores (adjusted M = 750.043, SE = 33.218) relative to participants receiving sham tDCS (adjusted M = 880.87, SE = 33.22), F1,24 = 7.51, P = 0.011, partial η2 = 0.24. An identical ANCOVA, with post-tDCS Stroop RT for incongruent trials as the dependent variable, tDCS condition as the independent variable, and pre-tDCS Stroop RT scores for neutral and incongruent trials as covariates revealed that participants allocated to the ALCR tDCS condition exhibited statistically significantly faster Stroop RT scores (adjusted M = 908.48, SE = 46.53) relative to participants allocated to the sham tDCS condition (adjusted M = 1072.41, SE = 46.53), F1,24 = 6.01, P = 0.022, partial η2 = 0.20. Results mirror the pattern of effects using change scores.
Conflict of Interest
None declared.
References
- Allom V, Mullan BA. Hagger MS. Does inhibitory control training improve behavior regulation? A meta-analysis. Perth, Australia: Curtin University; 2015. Unpublished manuscript. [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, et al. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend. 2008;92:55–60. doi: 10.1016/j.drugalcdep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Zaghi S, Villani AB, Fecteau S, Pascual-Leone A. Fregni F. Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC) Drug Alcohol Depend. 2010;112:220–225. doi: 10.1016/j.drugalcdep.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, Cedernaes J. Schiöth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS ONE. 2013;8:e60393. doi: 10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR. Vogt BA. The multi-source interference task: validation study with fMRI in individual subjects. Mol. Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR. Sahakian BJ. The neuropsychiatry of impulsivity. Curr. Opin. Psychiatry. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- Coderre EL. van Heuven WJB. Modulations of the executive control network by stimulus onset asynchrony in a Stroop task. BMC Neurosci. 2013;14:18. doi: 10.1186/1471-2202-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery CA, Hueckel-Weng R, Birbaumer N. Plewnia C. Enhancement of planning ability by transcranial direct current stimulation. J. Neurosci. 2009;29:7271–7277. doi: 10.1523/JNEUROSCI.0065-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer FN. Same and different judgments for word-color pairs with “irrelevant” words or colors: evidence for word-code comparisons. J. Exp. Psychol. 1973;98:102–108. doi: 10.1037/h0034278. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Pascual-Leone A, Zald DH, Liguori P, Theoret H, Boggio PS, et al. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. J. Neurosci. 2007;27:6212–6218. doi: 10.1523/JNEUROSCI.0314-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nat. Neurosci. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A. Boggio P. Cortical stimulation of the prefrontal cortex with transcranial Direct Current Stimulation reduces cue-provoked smoking craving: a randomised, sham controlled study. J. Clin. Psychiatry. 2008;69:32–40. doi: 10.4088/jcp.v69n0105. [DOI] [PubMed] [Google Scholar]
- Friese M, Hofmann W. Wänke M. When impulses take over: moderated predictive validity of explicit and implicit attitude measures in predicting food choice and consumption behaviour. Br. J. Soc. Psychol. 2008;47:397–419. doi: 10.1348/014466607X241540. [DOI] [PubMed] [Google Scholar]
- Friese M, Binder J, Luechinger R, Boesiger P. Rasch B. Suppressing emotions impairs subsequent Stroop performance and reduces prefrontal brain activation. PLoS ONE. 2013;8:e60385. doi: 10.1371/journal.pone.0060385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K. On conceptualizing self-control as more than the effortful inhibition of impulses. Pers. Soc. Psychol. Rev. 2011;15:352–366. doi: 10.1177/1088868311411165. [DOI] [PubMed] [Google Scholar]
- Glascher J, Hampton AN. O'Doherty JP. Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cereb. Cortex. 2009;19:483–495. doi: 10.1093/cercor/bhn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri R, Nedekoorn C. Jansen A. The interaction between impulsivity and a varied food environment: its influence on food intake and overweight. Int. J. Obes. 2008;32:708–714. doi: 10.1038/sj.ijo.0803770. [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Nedekoorn C. Jansen A. Disinhibition is easier learned than inhibition: the effects of (dis)inhibition training on food intake. Appetite. 2012;59:96–99. doi: 10.1016/j.appet.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Hagger MS. Chatzisarantis NLD. The sweet taste of success: the presence of glucose in the oral cavity moderates the depletion of self-control resources. Pers. Soc. Psychol. Bull. 2013;39:27–41. doi: 10.1177/0146167212459912. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Wood C, Stiff C. Chatzisarantis NLD. Ego depletion and the strength model of self-control: a meta-analysis. Psychol. Bull. 2010;136:495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Leung CM, Leaver E, Esser K, te Pas N, Keatley DA, et al. Cue-induced smoking urges deplete cigarette smokers’ self-control resources. Ann. Behav. Med. 2013a;46:394–400. doi: 10.1007/s12160-013-9520-8. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Panetta G, Leung C-M, Wong GG, Wang JCK, Chan DK-C, et al. Chronic inhibition, self-control and eating behavior: test of a ‘resource depletion’ model. PLoS ONE. 2013b;8:e76888. doi: 10.1371/journal.pone.0076888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF. Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Harvey N. Depletable resources: necessary, in need of fair treatment, and multi-functional. Behav. Brain Sci. 2012;36:689–690. doi: 10.1017/S0140525X13001015. [DOI] [PubMed] [Google Scholar]
- Heatherton TF. Neuroscience of self and self-regulation. Annu. Rev. Psychol. 2011;62:363–390. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF. Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn. Sci. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgcock WM, Vohs KD. Rao AR. Reducing self-control depletion effects through enhanced sensitivity to implementation: evidence from fMRI and behavioral studies. J. Consum. Psychol. 2012;22:486–495. [Google Scholar]
- Hofmann W, Friese M. Strack F. Impulse and self-control from a dual-systems perspective. Pers. Psychol. Sci. 2009;4:162–176. doi: 10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Schmeichel BJ. Baddeley AD. Executive functions and self-regulation. Trends Cogn. Sci. 2012;16:174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Houben K, Nederkoorn C, Wiers RW. Jansen A. Resisting temptation: decreasing alcohol-related affect and drinking behavior by training response inhibition. Drug Alcohol Depend. 2011;116:132–136. doi: 10.1016/j.drugalcdep.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Hyafil A, Summerfield C. Koechlin E. Two mechanisms for task switching in the prefrontal cortex. J. Neurosci. 2009;29:5135–5142. doi: 10.1523/JNEUROSCI.2828-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Koslowsky M. Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp. Brain Res. 2012;216:1–10. doi: 10.1007/s00221-011-2891-9. [DOI] [PubMed] [Google Scholar]
- Jeon SY. Han SJ. Improvement of the working memory and naming by transcranial direct current stimulation. Ann. Rehabil. Med. 2012;35:585–595. doi: 10.5535/arm.2012.36.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Guerrieri R, Fernie G, Cole J, Goudie A. Field M. The effects of priming restrained versus disinhibited behaviour on alcohol-seeking in social drinkers. Drug Alcohol Depend. 2011;113:55–61. doi: 10.1016/j.drugalcdep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V. Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314:829–832. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- Kurzban R, Duckworth AL, Kable JW. Myers J. An opportunity cost model of subjective effort and task performance. Behav. Brain Sci. 2013;36:661–679. doi: 10.1017/S0140525X12003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N, Nitsche MA, Dileone M, Mazzone P, De Andrés-Arés J, Diaz-Jara L, et al. Transcranial direct current stimulation effects on I-wave activity in humans. J. Neurophysiol. 2011;105:2802–2810. doi: 10.1152/jn.00617.2010. [DOI] [PubMed] [Google Scholar]
- Leite J, Carvalho S, Fregni F, Boggio PS. Goncalves OF. The effects of cross-hemispheric Dorsolateral Prefrontal Cortex transcranial Direct Current Stimulation (tDCS) on task switching. Brain Stimul. 2012;6:660–667. doi: 10.1016/j.brs.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JR, Ramsey SE, Stuart GL, et al. Evaluation of a behavioral measure of risk-taking: the balloon analogue risk task (BART) J. Exp. Psychol. Appl. 2002;6:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ. Tannock R. Impulsivity and inhibitory control. Psychol. Sci. 1997;8:60–64. [Google Scholar]
- Lüscher C. Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb. Perspect. Biol. 2012;4:a005710. doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA. Carter CS. Dissociating the role of the dorso-lateral pre-frontal cortex lateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol. Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- MacLeod CM, Wenzel A. Rubin DC. The stroop task in cognitive research. In: Wenzel A, Rubin DC, editors; Cognitive methods and their application to clinical research. Washington, DC: American Psychological Association; 2005. pp. 17–40. [Google Scholar]
- Muraven M. Shmueli D. The self-control costs of fighting the temptation to drink. Psychol. Addict. Behav. 2006;20:154–160. doi: 10.1037/0893-164X.20.2.154. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Houben K, Hofmann W, Roefs A. Jansen A. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychol. 2010;29:389–393. doi: 10.1037/a0019921. [DOI] [PubMed] [Google Scholar]
- Nitsche MA. Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–133. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rebar AL, Loftus AM. Hagger MS. Cognitive control and the non-conscious regulation of health behavior. Front. Hum. Neurosci. 2015;9:122. doi: 10.3389/fnhum.2015.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KL. Hall DA. Examining a supramodal network for conflict processing: a systematic review and novel functional magnetic resonance Imaging data for related visual and auditory stroop tasks. J. Cogn. Neurosci. 2008;20:1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- Steinbeis N, Bernhardt BC. Singer T. Impulse control and underlying functions of the left DLPFC mediate age-related and age-independent individual differences in strategic social behaviour. Neuron. 2012;73:1040–1051. doi: 10.1016/j.neuron.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Todd J. Mullan BA. The role of self-monitoring and response inhibition in improving sleep behaviours. Int. J. Behav. Med. 2013;21:470–477. doi: 10.1007/s12529-013-9328-8. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt MA, De Raedt R, Baeken C, Leyman L. D'Haenen H. The influence of rTMS over the left dorsolateral prefrontal cortex on Stroop task performance. Exp. Brain Res. 2006;169:279–282. doi: 10.1007/s00221-005-0344-z. [DOI] [PubMed] [Google Scholar]
- Veling HP, Aarts H. Papies EK. Using stop signals to inhibit dieters’ responses toward palatable foods. Behav. Res. Ther. 2011;49:771–780. doi: 10.1016/j.brat.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Veling HP, Aarts H. Stroebe W. Stop signals decrease choices for palatable foods through decreased food evaluation. Front. Psychol. 2013a;4:875. doi: 10.3389/fpsyg.2013.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veling HP, Aarts H. Stroebe W. Using stop signals to reduce impulsive choices for palatable unhealthy foods. Br. J. Health Psychol. 2013b;18:354–368. doi: 10.1111/j.2044-8287.2012.02092.x. [DOI] [PubMed] [Google Scholar]
- Vohs KD. Heatherton TF. Self-regulatory failure: a resource-depletion approach. Psychol. Sci. 2000;11:249–254. doi: 10.1111/1467-9280.00250. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA. Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat. Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Qiu J. Zhang QL. The dissociation of neural circuits in a Stroop task. NeuroReport. 2009;20:674–678. doi: 10.1097/WNR.0b013e32832a0a10. [DOI] [PubMed] [Google Scholar]
- Zaghi S, Acar M, Hultgren B, Boggio PS. Fregni F. Non-invasive brain stimulation with low-intensity electrical currents: Putative mechanisms of action for direct and alternating current stimulation. Neuroscientist. 2010;16:285–307. doi: 10.1177/1073858409336227. [DOI] [PubMed] [Google Scholar]


