Abstract
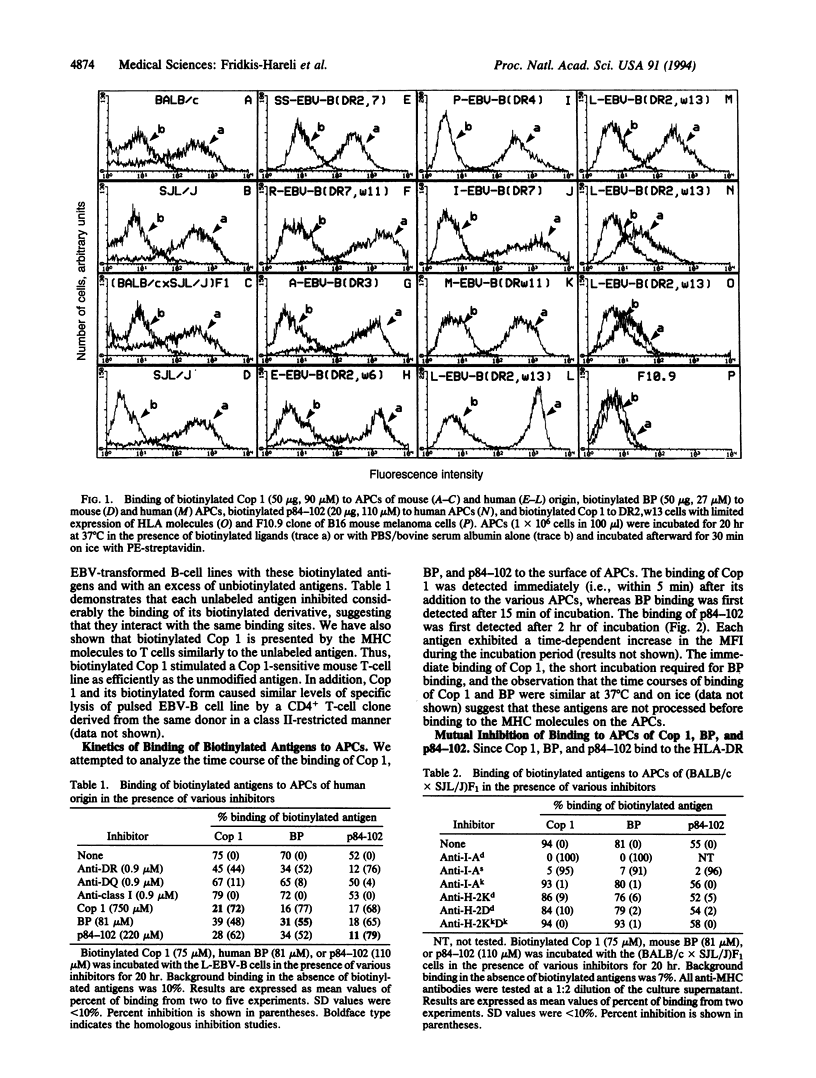
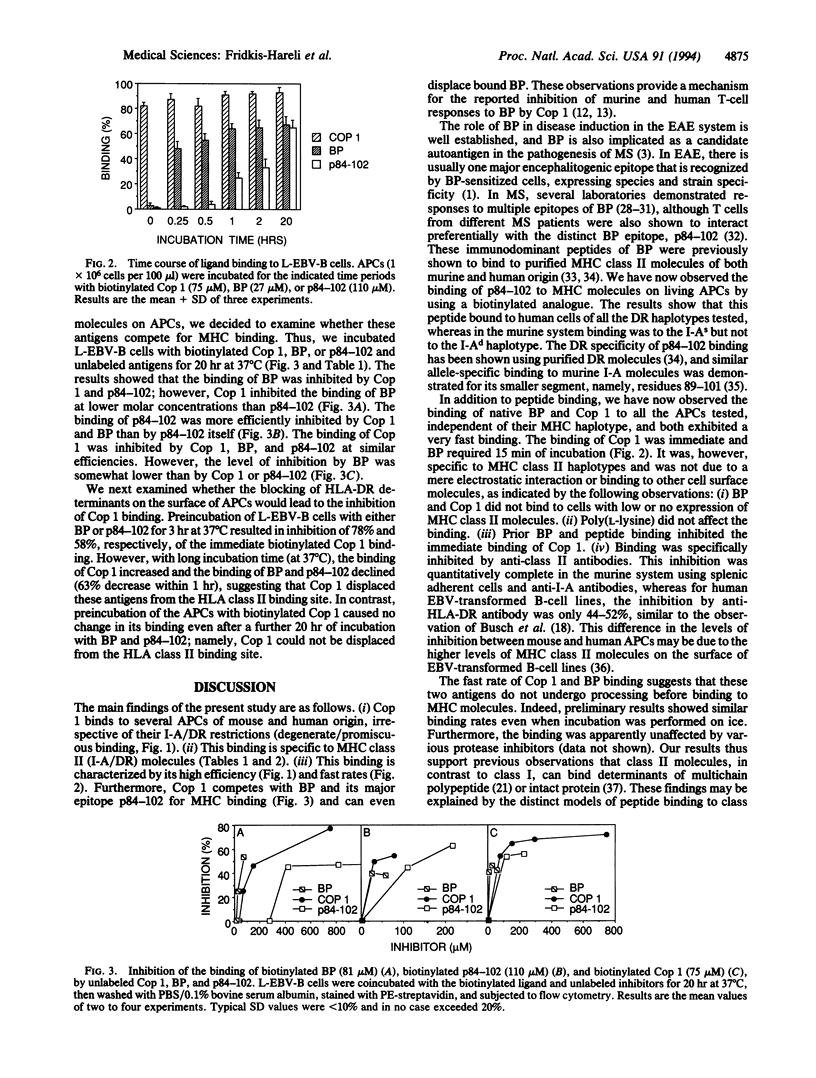
Copolymer 1 (Cop 1) is a synthetic basic random copolymer of amino acids that has been shown to be effective in suppression of experimental allergic encephalomyelitis and is being tested as a candidate drug for multiple sclerosis. It has been previously demonstrated that Cop 1 is immunologically cross-reactive with the autoantigen myelin basic protein (BP) and competitively inhibits the response to BP of T-cell lines and clones of different major histocompatibility complex (MHC) restrictions, of both mouse and human origin. In the present study we demonstrated the direct binding of Cop 1, using its biotinylated derivative, to MHC molecules on living antigen-presenting cells. Binding of biotinylated BP and peptide p84-102 (an immunodominant epitope of BP) was also demonstrated. Cop 1 and BP bound in a promiscuous manner to different types of antigen-presenting cells of various H-2 and HLA haplotypes. The specificity of the binding was confirmed by its inhibition with either the relevant anti-MHC class II antibodies or unlabeled analogs. Cop 1 exhibited the most extensive and fast binding to antigen-presenting cells. In addition, Cop 1 inhibited the binding of biotinylated derivatives of BP and of p84-102 to the MHC class II molecules and even displaced these antigens when already bound. Thus, these results suggest that Cop 1 indeed competes with BP for MHC binding and, thereby, inhibits T-cell responses to BP. The binding of Cop 1 to different DR alleles, probably because of its multiple MHC binding motifs, may indicate its potential as a broad-spectrum drug for multiple sclerosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni R., Teitelbaum D., Arnon R. T suppressor hybridomas and interleukin-2-dependent lines induced by copolymer 1 or by spinal cord homogenate down-regulate experimental allergic encephalomyelitis. Eur J Immunol. 1993 Jan;23(1):17–25. doi: 10.1002/eji.1830230105. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Brenner T., Timore Y., Wirguin I., Abramsky O., Steinitz M. In vitro synthesis of antibodies to acetylcholine receptor by Epstein-Barr virus-stimulated B-lymphocytes derived from patients with myasthenia gravis. J Neuroimmunol. 1989 Oct;24(3):217–222. doi: 10.1016/0165-5728(89)90119-7. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., Strominger J. L., Wiley D. C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993 Jul 1;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- Busch R., Strang G., Howland K., Rothbard J. B. Degenerate binding of immunogenic peptides to HLA-DR proteins on B cell surfaces. Int Immunol. 1990;2(5):443–451. doi: 10.1093/intimm/2.5.443. [DOI] [PubMed] [Google Scholar]
- Ceppellini R., Frumento G., Ferrara G. B., Tosi R., Chersi A., Pernis B. Binding of labelled influenza matrix peptide to HLA DR in living B lymphoid cells. Nature. 1989 Jun 1;339(6223):392–394. doi: 10.1038/339392a0. [DOI] [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Gorga J. C., Vignali D. A., Lane W. S., Strominger J. L. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993 Jul 1;178(1):27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y. K., Vainiene M., Whitham R., Bourdette D., Chou C. H., Hashim G., Offner H., Vandenbark A. A. Response of human T lymphocyte lines to myelin basic protein: association of dominant epitopes with HLA class II restriction molecules. J Neurosci Res. 1989 Jun;23(2):207–216. doi: 10.1002/jnr.490230211. [DOI] [PubMed] [Google Scholar]
- GREEN N. M. A SPECTROPHOTOMETRIC ASSAY FOR AVIDIN AND BIOTIN BASED ON BINDING OF DYES BY AVIDIN. Biochem J. 1965 Mar;94:23C–24C. doi: 10.1042/bj0940023c. [DOI] [PubMed] [Google Scholar]
- Govaerts A., Gony J., Martin-Mondiére C., Poirier J. C., Schmid M., Schuller E., Degos J. D., Dausset J. HLA and multiple sclerosis: population and families study. Tissue Antigens. 1985 Apr;25(4):187–199. doi: 10.1111/j.1399-0039.1985.tb00436.x. [DOI] [PubMed] [Google Scholar]
- Hammer J., Takacs B., Sinigaglia F. Identification of a motif for HLA-DR1 binding peptides using M13 display libraries. J Exp Med. 1992 Oct 1;176(4):1007–1013. doi: 10.1084/jem.176.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld H., Teitelbaum D., Arnon R., Sela M. Basic encephalitogenic protein: A simplified purification on sulphoethyl-sephadex. FEBS Lett. 1970 May 1;7(4):317–320. doi: 10.1016/0014-5793(70)80193-4. [DOI] [PubMed] [Google Scholar]
- Jersild C., Fog T., Hansen G. S., Thomsen M., Svejgaard A., Dupont B. Histocompatibility determinants in multiple sclerosis, with special reference to clinical course. Lancet. 1973 Dec 1;2(7840):1221–1225. doi: 10.1016/s0140-6736(73)90970-7. [DOI] [PubMed] [Google Scholar]
- Kubitscheck U., Levi R., Horwitz R. J., Arnon R., Pecht I. Peptide binding to class I molecules of the major histocompatibility complex on the surface of living target cells. Scand J Immunol. 1992 Aug;36(2):341–348. doi: 10.1111/j.1365-3083.1992.tb03107.x. [DOI] [PubMed] [Google Scholar]
- Lando Z., Teitelbaum D., Arnon R. Effect of cyclophosphamide on suppressor cell activity in mice unresponsive to EAE. J Immunol. 1979 Nov;123(5):2156–2160. [PubMed] [Google Scholar]
- Liblau R., Tournier-Lasserve E., Maciazek J., Dumas G., Siffert O., Hashim G., Bach M. A. T cell response to myelin basic protein epitopes in multiple sclerosis patients and healthy subjects. Eur J Immunol. 1991 Jun;21(6):1391–1395. doi: 10.1002/eji.1830210610. [DOI] [PubMed] [Google Scholar]
- Marrosu M. G., Muntoni F., Murru M. R., Spinicci G., Pischedda M. P., Goddi F., Cossu P., Pirastu M. Sardinian multiple sclerosis is associated with HLA-DR4: a serologic and molecular analysis. Neurology. 1988 Nov;38(11):1749–1753. doi: 10.1212/wnl.38.11.1749. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B. Automated synthesis of peptides. Science. 1965 Oct 8;150(3693):178–185. doi: 10.1126/science.150.3693.178. [DOI] [PubMed] [Google Scholar]
- Mozes E., Dayan M., Zisman E., Brocke S., Licht A., Pecht I. Direct binding of a myasthenia gravis related epitope to MHC class II molecules on living murine antigen-presenting cells. EMBO J. 1989 Dec 20;8(13):4049–4052. doi: 10.1002/j.1460-2075.1989.tb08588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan D., Arrhenius T., Sidney J., Del Guercio M. F., Albertson M., Wall M., Oseroff C., Southwood S., Colón S. M., Gaeta F. C. On the interaction of promiscuous antigenic peptides with different DR alleles. Identification of common structural motifs. J Immunol. 1991 Oct 15;147(8):2663–2669. [PubMed] [Google Scholar]
- Ota K., Matsui M., Milford E. L., Mackin G. A., Weiner H. L., Hafler D. A. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990 Jul 12;346(6280):183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P., Tan A., Termijtelen A., Demotz S., Corradin G., Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989 Dec;19(12):2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- Paterson P. Y., Day E. D. Current perspectives of neuroimmunologic disease: multiple sclerosis and experimental allergic encephalomyelitis (1,2). Clin Immunol Rev. 1981;1(4):581–697. [PubMed] [Google Scholar]
- Pette M., Fujita K., Kitze B., Whitaker J. N., Albert E., Kappos L., Wekerle H. Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology. 1990 Nov;40(11):1770–1776. doi: 10.1212/wnl.40.11.1770. [DOI] [PubMed] [Google Scholar]
- Porgador A., Feldman M., Eisenbach L. H-2Kb transfection of B16 melanoma cells results in reduced tumourigenicity and metastatic competence. J Immunogenet. 1989 Aug-Oct;16(4-5):291–303. doi: 10.1111/j.1744-313x.1989.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Richert J. R., Robinson E. D., Deibler G. E., Martenson R. E., Dragovic L. J., Kies M. W. Evidence for multiple human T cell recognition sites on myelin basic protein. J Neuroimmunol. 1989 Jun;23(1):55–66. doi: 10.1016/0165-5728(89)90073-8. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Busch R., Howland K., Bal V., Fenton C., Taylor W. R., Lamb J. R. Structural analysis of a peptide--HLA class II complex: identification of critical interactions for its formation and recognition by T cell receptor. Int Immunol. 1989;1(5):479–486. doi: 10.1093/intimm/1.5.479. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Gefter M. L. Interactions between immunogenic peptides and MHC proteins. Annu Rev Immunol. 1991;9:527–565. doi: 10.1146/annurev.iy.09.040191.002523. [DOI] [PubMed] [Google Scholar]
- Speck S. H., Strominger J. L. Epstein-Barr virus transformation. Prog Nucleic Acid Res Mol Biol. 1987;34:189–207. [PubMed] [Google Scholar]
- Teitelbaum D., Aharoni R., Arnon R., Sela M. Specific inhibition of the T-cell response to myelin basic protein by the synthetic copolymer Cop 1. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9724–9728. doi: 10.1073/pnas.85.24.9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum D., Aharoni R., Sela M., Arnon R. Cross-reactions and specificities of monoclonal antibodies against myelin basic protein and against the synthetic copolymer 1. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9528–9532. doi: 10.1073/pnas.88.21.9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum D., Meshorer A., Hirshfeld T., Arnon R., Sela M. Suppression of experimental allergic encephalomyelitis by a synthetic polypeptide. Eur J Immunol. 1971 Aug;1(4):242–248. doi: 10.1002/eji.1830010406. [DOI] [PubMed] [Google Scholar]
- Teitelbaum D., Milo R., Arnon R., Sela M. Synthetic copolymer 1 inhibits human T-cell lines specific for myelin basic protein. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):137–141. doi: 10.1073/pnas.89.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum D., Webb C., Bree M., Meshorer A., Arnon R., Sela M. Suppression of experimental allergic encephalomyelitis in Rhesus monkeys by a synthetic basic copolymer. Clin Immunol Immunopathol. 1974 Nov;3(2):256–262. doi: 10.1016/0090-1229(74)90012-9. [DOI] [PubMed] [Google Scholar]
- Teitelbaum D., Webb C., Meshorer A., Arnon R., Sela M. Suppression by several synthetic polypeptides of experimental allergic encephalomyelitis induced in guinea pigs and rabbits with bovine and human basic encephalitogen. Eur J Immunol. 1973 May;3(5):273–279. doi: 10.1002/eji.1830030505. [DOI] [PubMed] [Google Scholar]
- Valli A., Sette A., Kappos L., Oseroff C., Sidney J., Miescher G., Hochberger M., Albert E. D., Adorini L. Binding of myelin basic protein peptides to human histocompatibility leukocyte antigen class II molecules and their recognition by T cells from multiple sclerosis patients. J Clin Invest. 1993 Feb;91(2):616–628. doi: 10.1172/JCI116242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall M., Southwood S., Sidney J., Oseroff C., del Guericio M. F., Lamont A. G., Colón S. M., Arrhenius T., Gaeta F. C., Sette A. High affinity for class II molecules as a necessary but not sufficient characteristic of encephalitogenic determinants. Int Immunol. 1992 Jul;4(7):773–777. doi: 10.1093/intimm/4.7.773. [DOI] [PubMed] [Google Scholar]
- Webb C., Teitelbaum D., Arnon R., Sela M. In vivo and in vitro immunological cross-reactions between basic encephalitogen and synthetic basic polypeptides capable of suppressing experimental allergic encephalomyelitis. Eur J Immunol. 1973 May;3(5):279–286. doi: 10.1002/eji.1830030506. [DOI] [PubMed] [Google Scholar]
- Wraith D. C., Smilek D. E., Mitchell D. J., Steinman L., McDevitt H. O. Antigen recognition in autoimmune encephalomyelitis and the potential for peptide-mediated immunotherapy. Cell. 1989 Oct 20;59(2):247–255. doi: 10.1016/0092-8674(89)90287-0. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Weiner H. L., Hafler D. A. T-cell recognition of myelin basic protein. Immunol Today. 1991 Aug;12(8):277–282. doi: 10.1016/0167-5699(91)90126-E. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- Zisman E., Dayan M., Sela M., Mozes E. Ia-antigen-T-cell interactions for a thymus-independent antigen composed of D amino acids. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):994–998. doi: 10.1073/pnas.90.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisman E., Sela M., Mozes E. Direct binding of a synthetic multichain polypeptide to class II major histocompatibility complex molecules on antigen-presenting cells and stimulation of a specific T-cell line require processing of the polypeptide. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9738–9742. doi: 10.1073/pnas.88.21.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]


