Abstract
Critically ill neonates are at high risk for acute kidney injury (AKI). Renal supportive therapy (RST) can be an important tool for supporting critically ill neonates with AKI, particularly in cases of oliguria and fluid overload. There are few reports of RST for management of oligo-anuric AKI in the extremely low-birth-weight infant weighing <1000 g. We report successful provision of peritoneal dialysis (PD) to an 830-g neonate with oligo-anuric AKI through adaptation of a standard pediatric acute PD catheter.
Keywords: acute kidney injury, extremely low-birth-weight infant, peritoneal dialysis, renal supportive therapy
Background
Neonates, especially very low-birth-weight infants [1], are at high risk for acute kidney injury (AKI), and AKI is an independent predictor of morbidity and mortality in this patient population [1–5]. When used in neonates, renal supportive therapy (RST) is often considered a last resort when all other medical management has failed. The decision to offer RST to the smallest of patients is complicated by a number of challenges including limited availability of appropriately sized dialysis catheters, lack of machines designed for neonates, technical difficulties associated with access placement in small infants, as well as individual physician practices, biases and beliefs. Limited available literature documenting the use of peritoneal dialysis (PD) in extremely low-birth-weight (ELBW) infants describes uniformly high rates of technical complications (25–60%) and mortality (>50%) [6–8]. For these reasons, practitioners may defer RST in favor of diuretic therapy and nutritional restriction in ELBW neonates or offer no dialytic support at all. We report the successful provision of PD to an 830-g premature infant with oligo-anuric AKI and deteriorating respiratory status using a commercially available temporary PD catheter.
Case report
The patient was a male infant born at 28 and 3/7 weeks gestation weighing 830 g. He was Twin B of a monochorionic twin gestation, and the donor in a twin–twin transfusion syndrome. The pregnancy was complicated by oligohydramnios, worsening cardiac function and hydrops in Twin A necessitating preterm delivery via cesarean section. Twin B's Apgar scores were 8 and 8, at 1 and 5 min, respectively; however, he developed respiratory distress and required intubation. He was placed on high-frequency oscillatory ventilation.
The infant was anuric on Day of life (DOL) 1. He received furosemide and aminophylline to stimulate urine output without effect. Metolazone was added, but he remained anuric. A renal ultrasound demonstrated echogenic kidneys with normal Doppler flow and an empty bladder. By DOL 3, the infant's weight had risen to 1050 g, a 27% increase from birth weight. Laboratory studies were significant for serum sodium 128 mEq/L (reference range, 135–145 mEq/L), serum creatinine 2.3 mg/dL (reference range, 0.2–0.9 mg/dL) and blood urea nitrogen (BUN) 69 mg/dL (reference range, 10–20 mg/dL).
The infant was transferred to the University of Iowa Children's Hospital Neonatal Intensive Care Unit for respiratory and renal management. Diuretic therapy was discontinued upon arrival given lack of effect. Total fluids were restricted to a volume of 75 mL/kg/day for the first 24 h post-transfer with further restriction to 60 mL/kg/day and 1 g/kg/day of protein on DOL 4–5 due to worsening fluid overload and significant decline in respiratory status. The infant's renal parameters indicated severe anuric renal failure, with a BUN and creatinine of 87 and 2.7 mg/dL, respectively.
On DOL 5, the infant's weight was 1096 g, indicating a 32% fluid weight gain from birth. Given the dire clinical picture and the likelihood of death without intervention, the neonatology, pediatric surgery and pediatric nephrology teams reviewed the risks and benefits of RST with the patient's family and devised a PD plan for the infant.
Procedure
The primary technical challenge for initiating PD in this ELBW infant was that standard cuffed PD catheters, with lengths from 31 to 39 cm, are too large for an ELBW infant's small abdominal domain. Acute PD catheters with 8 cm catheter lengths represent the smallest commercially available PD catheter. However, these are likely still too large for an 800-g infant when placed in the standard fashion via an infra-umbilical stab incision with the catheter directed into the pelvis. To use a true PD catheter rather than improvised equipment (e.g. large-bore intravenous cannula) [9] that would be prone to complications, we thus considered a novel placement approach using an acute PD catheter inserted into the left upper quadrant.
An 8.5-French, 8-cm commercial temporary PD catheter (Pediatric Peritoneal Dialysis Set—Cook Medical) was placed at the bedside by a pediatric surgeon. A small stab incision was made in the left upper quadrant. A dilator was then placed through the stab incision into the abdomen. Using the Seldinger technique, the PD catheter was exchanged for the dilator and directed into the pelvis such that the catheter remained straight. A purse-string suture was used to secure a watertight closure of the posterior rectus sheath. The subcutaneous tissues and skin were closed with running suture to prevent leakage of peritoneal fluid and permit initiation of PD immediately. The exit site was closed around the catheter and dressed. Catheter placement was confirmed radiographically (see Figure 1a and b) and with successful instillation and aspiration of saline from the catheter. See Figure 2 for photograph of catheter location.
Fig. 1.
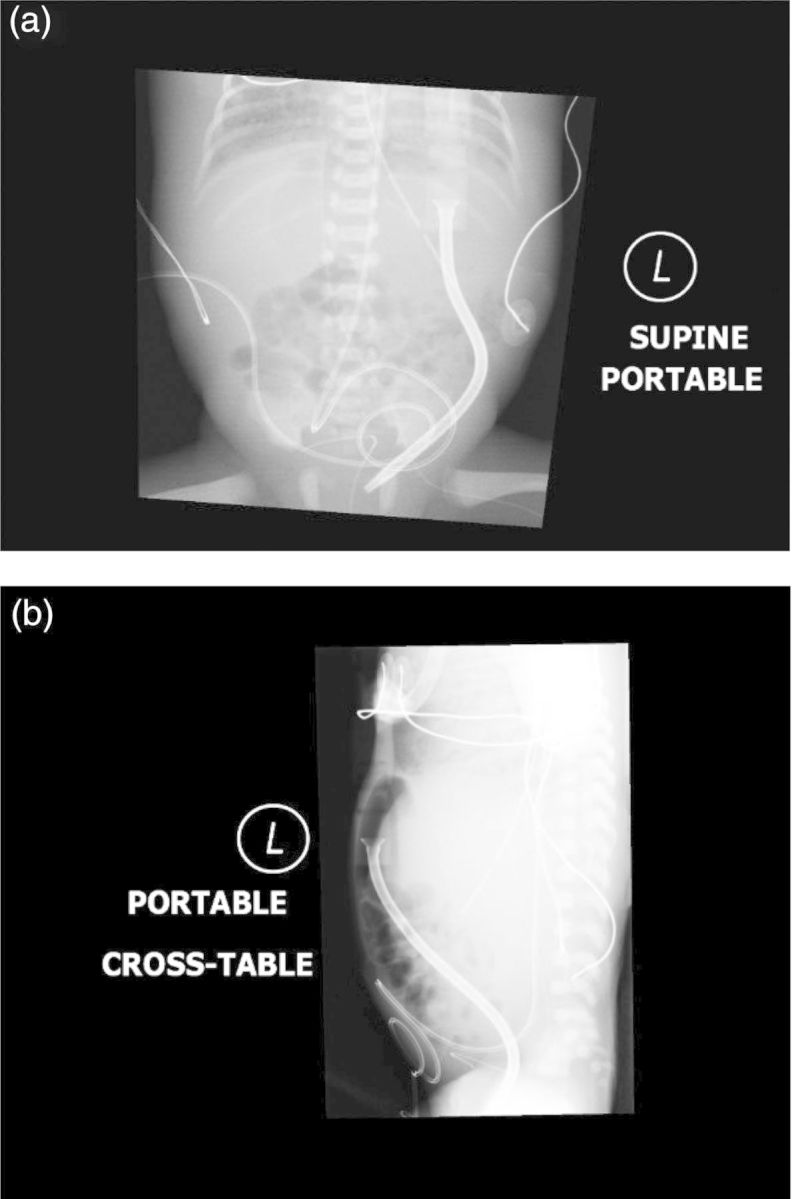
(a) AP X-ray demonstrating PD catheter placement coursing from the left upper quadrant into the left paracolic gutter. (b) Lateral X-ray demonstrating PD catheter placement coursing from the left upper quadrant into the left paracolic gutter.
Fig. 2.
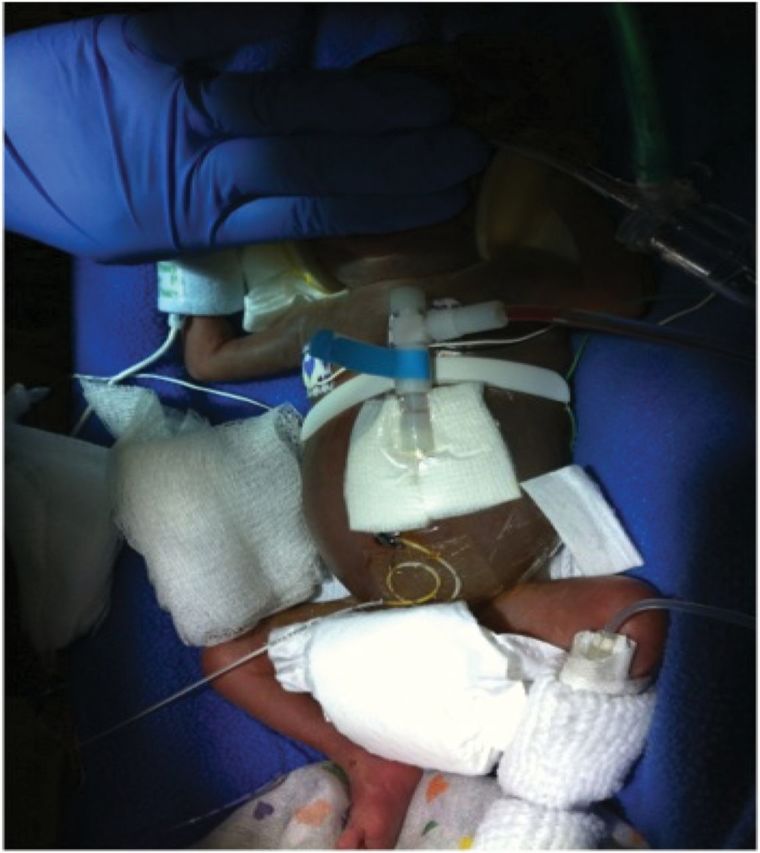
Photograph of PD catheter placement.
Following catheter placement, manual PD using the Dialy-Nate® system was initiated with 16 mL volume dwells (∼20 mL/kg based on birth weight) of 45 min using 1.5% Dianeal® for 24 cycles per day. The dextrose concentration of the dialysate was later increased to 2.5% to optimize ultrafiltration. The infant developed hypotension, so dwell times were decreased to 30 min followed by 25-min drain periods, resulting in better blood pressures while maintaining adequate ultrafiltration and clearance. Within 72 h of initiating PD, the infant achieved a net ultrafiltration of 4–6 mL/pass.
With successful dialysis, uremia improved; BUN decreased from 87 to 45 mg/dL. After 3 days, total fluid intake was liberalized to 120 mL/kg/day for venous nutrition. Dietary protein was liberalized to 3.5 g per kilogram per day. Enteral feedings were started on DOL 8. Weight decreased to 1024 g from a peak of 1092 g over the course of 10 days.
The infant was transitioned from high frequency to conventional ventilation on DOL 13. By DOL 15, urine output had improved to 3–4 mL/kg/hr, and the infant maintained appropriate fluid balance during trials off PD. PD was discontinued on DOL 21. The catheter was removed on DOL 28. Adequate urine output was maintained during the remainder of his hospital admission without diuretic. Renal labs improved prior to discharge to a BUN of 28 mg/dL and a serum creatinine of 0.9 mg/dL. No acute infectious complications were experienced during the use of PD. There were no episodes of PD catheter leak.
The infant was discharged to home on DOL 105. He is being followed by pediatric nephrology for the monitoring of his chronic kidney disease. He does not require RST or diuretic therapy. Current laboratory studies demonstrate a serum creatinine of 0.5 mg/dL at age 16 months, suggesting an estimated glomerular filtration rate of 61 mL/min/1.73 m2 by the modified Schwartz equation. He no longer requires supplemental oxygen or other respiratory support.
Discussion
To our knowledge, our case report includes the smallest infant with whom PD was successful using a standard, commercially available PD catheter. RST was indicated given severe oligo-anuric AKI resistant to medical management, fluid overload resulting in respiratory and hemodynamic compromise and inability to provide adequate nutrition secondary to fluid restriction. Use of RST in neonates is often limited by lack of size-specific equipment. PD was chosen for RST due to the lack of appropriately sized vascular catheters for a patient of such small size. Due to our infant's extremely small size and severe fluid overload, novel PD catheter placement was required. His initial dialysis prescription also required adaptation with increased initial fill volumes of 20 mL/kg to overcome the comparatively large amount of dead space within dialysis tubing (a catheter volume of 0.4 mL and a connecting tube volume of 3.0 mL).
Limited existing literature documents high mortality rates in ELBW infants treated with PD, though the high rates in some reports are likely related to the underlying medical conditions. For example, two case series using PD in neonates each reported >50% mortality but included many infants with inborn errors of metabolism [6, 7]. Continuous veno-venous hemodialysis is the preferred therapy for patients with this condition because of its superior efficacy [10]; inefficient clearance with PD is not adequate for rapid removal of ammonia in these cases. Similarly, one group reported a 75% mortality rate in the use of PD for infants with necrotizing enterocolitis [11], a condition associated with high mortality rates as well as one which would be considered a relative contraindication to the use of PD [12] because of the inflammation in the abdomen and high risk for infection.
In addition to high mortality rates, the available literature describes high rates of complications such as catheter leak and peritonitis during the use of PD in small infants, likely related to the lack of size-specific equipment for these patients. Use of varying catheter types, both commercial and improvised, for PD in ELBW neonates has been described. Two larger case series of PD in neonates (BW range, 1–4 kg) report technical success using rigid catheters in the infra-umbilical position [6, 7]. The patients in these series were all larger than ours. Oyachi et al. describe successful use of a flexible 10-French Blake silicone drain in a 40-week term infant requiring PD [13]. Yu et al. detail novel use of a vascular catheter placed in the right upper quadrant; however, high rates of catheter leak, poor drainage, and kinking were noted [14]. Similarly, use of a large-bore intravenous cannula has been described; however, again this improvised device was prone to malfunction with leak and kinking [9]. To our knowledge, this case illustrates the first time a standard, temporary PD catheter has been placed successfully via a left upper abdominal quadrant insertion site facilitating uncoiled extension into the left paracolic gutter in an ELBW infant.
Early initiation of RST for AKI and fluid overload in neonates may have important short- and long-term systemic effects [15]. Adult and experimental data suggest that mortality due to AKI may be related to multi-organ functional and transcriptional changes, especially in the lungs [16–19]. AKI is implicated in the development of bronchopulmonary dysplasia due to pulmonary edema from volume overload and the associated elevation in pro-inflammatory cytokines [20]. The adult critical care literature demonstrates that critically ill patients with AKI are more difficult to wean from mechanical ventilation [21]. These findings bolster the need for early fluid removal and treatment of AKI in neonates given the implications for sequelae of chronic lung disease in this vulnerable population. Herein, the neonatology team was able to transition our infant from high frequency to conventional ventilation within 1 week of dialysis initiation.
Our infant's severely fluid restricted state prior to RST initiation eliminated any opportunity to provide nutrition without further fluid accumulation. Adequate nutritional support may be unintentionally deferred in critically ill patients due to fluid restriction, preparation for surgical/procedural interventions and/or concern for feeding intolerance [22]. The resultant malnutrition in the setting of AKI is associated with increased morbidity and mortality [23, 24]. Nutritional deficit, specifically protein debt, is linked to higher risk for infection, reduced wound healing and increased need for mechanical ventilation [25]. Provision of adequate nutritional support to critically ill patients is a major benefit of RST.
Conclusion
The smallest of patients are at high risk for morbidity and mortality secondary to AKI, yet there remains a dearth of clinical equipment and expertise available to provide adequate RST for these patients. Technical adaptation is required for placement of PD catheters in ELBW infants; however, renal recovery following severe oligo-anuric AKI is possible and can be followed by excellent growth, development and improvement in lung function. These infants should not be excluded from consideration for initiation of RST because of their small size.
Acknowledgements
The preparation of this case report did not require any outside funding or grant support.
Conflict of interest statement. The authors have no conflicts of interest to disclose as part of this case report. The information presented in this paper has not been published previously in whole or part.
References
- 1.Koralkar R, Ambalavanan N, Levitan EB, et al. Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res. 2011;69:354–358. doi: 10.1203/PDR.0b013e31820b95ca. [DOI] [PubMed] [Google Scholar]
- 2.Askenazi DJ, Koralkar R, Hundley HE, et al. Fluid overload and mortality are associated with acute kidney injury in sick near-term/term neonate. Pediatr Nephrol. 2013;28:661–666. doi: 10.1007/s00467-012-2369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selewski DT, Jordan BK, Askenazi DJ, et al. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J Pediatr. 2013;162:725–729. doi: 10.1016/j.jpeds.2012.10.002. e1. [DOI] [PubMed] [Google Scholar]
- 4.Askenazi DJ, Griffin R, McGwin G, et al. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr Nephrol. 2009;24:991–997. doi: 10.1007/s00467-009-1133-x. [DOI] [PubMed] [Google Scholar]
- 5.Gadepalli SK, Selewski DT, Drongowski RA, et al. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg. 2011;46:630–635. doi: 10.1016/j.jpedsurg.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Alparslan C, Yavascan O, Bal A, et al. The performance of acute peritoneal dialysis treatment in neonatal period. Ren Fail. 2012;34:1015–1020. doi: 10.3109/0886022X.2012.708378. [DOI] [PubMed] [Google Scholar]
- 7.Unal S, Bilgin L, Gunduz M, et al. The implementation of neonatal peritoneal dialysis in a clinical setting. J Matern Fetal Neonatal Med. 2012;25:2111–2114. doi: 10.3109/14767058.2012.665105. [DOI] [PubMed] [Google Scholar]
- 8.Genc G, Bicakci U, Gunaydin M, et al. Temporary peritoneal dialysis in newborns and children: a single-center experience over five years. Ren Fail. 2012;34:1058–1061. doi: 10.3109/0886022X.2012.715574. [DOI] [PubMed] [Google Scholar]
- 9.Stojanovic V, Bukarica S, Doronjski A, et al. Peritoneal dialysis in neonates with extremely low body weight at birth: new modality of using IV cannula for peritoneal access. Iran J Pediatr. 2013;23:718–720. [PMC free article] [PubMed] [Google Scholar]
- 10.Spinale JM, Laskin BL, Sondheimer N, et al. High-dose continuous renal replacement therapy for neonatal hyperammonemia. Pediatr Nephrol. 2013;28:983–986. doi: 10.1007/s00467-013-2441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canpolat FE, Yurdakok M, Yigit S, et al. Can peritoneal dialysis be used in preterm infants with gastrointestinal perforation? Pediatr Int. 2010;52:834–835. doi: 10.1111/j.1442-200X.2010.03114.x. [DOI] [PubMed] [Google Scholar]
- 12.Walters S, Porter C, Brophy PD. Dialysis and pediatric acute kidney injury: choice of renal support modality. Pediatr Nephrol. 2009;24:37–48. doi: 10.1007/s00467-008-0826-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oyachi N, Obana K, Kimura S, et al. Use of a flexible Blake(R) silicone drains for peritoneal dialysis in the neonatal intensive care unit. Pediatr Int. 2011;53:417–418. doi: 10.1111/j.1442-200X.2011.03366.x. [DOI] [PubMed] [Google Scholar]
- 14.Yu JE, Park MS, Pai KS. Acute peritoneal dialysis in very low birth weight neonates using a vascular catheter. Pediatr Nephrol. 2010;25:367–371. doi: 10.1007/s00467-009-1347-y. [DOI] [PubMed] [Google Scholar]
- 15.Phillips JB, Lovvorn JJ, Nye LH, et al. Characteristics, mortality, and outcome of acquired anasarca in the NICU. e-J Neonatol Res. 2012;2:126–129. [Google Scholar]
- 16.Kim do J, Park SH, Sheen MR, et al. Comparison of experimental lung injury from acute renal failure with injury due to sepsis. Respiration. 2006;73:815–824. doi: 10.1159/000095588. [DOI] [PubMed] [Google Scholar]
- 17.Faubel S. Pulmonary complications after acute kidney injury. Adv Chronic Kidney Dis. 2008;15:284–296. doi: 10.1053/j.ackd.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Hassoun HT, Santora R, et al. Organ crosstalk: the role of the kidney. Curr Opin Crit Care. 2009;15:481–487. doi: 10.1097/MCC.0b013e328332f69e. [DOI] [PubMed] [Google Scholar]
- 19.Doi K, Ishizu T, Fujita T, et al. Lung injury following acute kidney injury: kidney-lung crosstalk. Clin Exp Nephrol. 2011;15:464–470. doi: 10.1007/s10157-011-0459-4. [DOI] [PubMed] [Google Scholar]
- 20.Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med. 2008;358:1700–1711. doi: 10.1056/NEJMra0707601. [DOI] [PubMed] [Google Scholar]
- 21.Vieira JM, Jr., Castro I, Curvello-Neto A, et al. Effect of acute kidney injury on weaning from mechanical ventilation in critically ill patients. Crit Care Med. 2007;35:184–191. doi: 10.1097/01.CCM.0000249828.81705.65. [DOI] [PubMed] [Google Scholar]
- 22.Lambe C, Hubert P, Jouvet P, et al. A nutritional support team in the pediatric intensive care unit: changes and factors impeding appropriate nutrition. Clin Nutr. 2007;26:355–363. doi: 10.1016/j.clnu.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Fiaccadori E, Cremaschi E, Regolisti G. Nutritional assessment and delivery in renal replacement therapy patients. Semin Dial. 2011;24:169–175. doi: 10.1111/j.1525-139X.2011.00831.x. [DOI] [PubMed] [Google Scholar]
- 24.Wooley JA, Btaiche IF, Good KL. Metabolic and nutritional aspects of acute renal failure in critically ill patients requiring continuous renal replacement therapy. Nutr Clin Pract. 2005;20:176–191. doi: 10.1177/0115426505020002176. [DOI] [PubMed] [Google Scholar]
- 25.Fiaccadori E, Lombardi M, Leonardi S, et al. Prevalence and clinical outcome associated with preexisting malnutrition in acute renal failure: a prospective cohort study. J Am Soc Nephrol. 1999;10:581–593. doi: 10.1681/ASN.V103581. [DOI] [PubMed] [Google Scholar]


