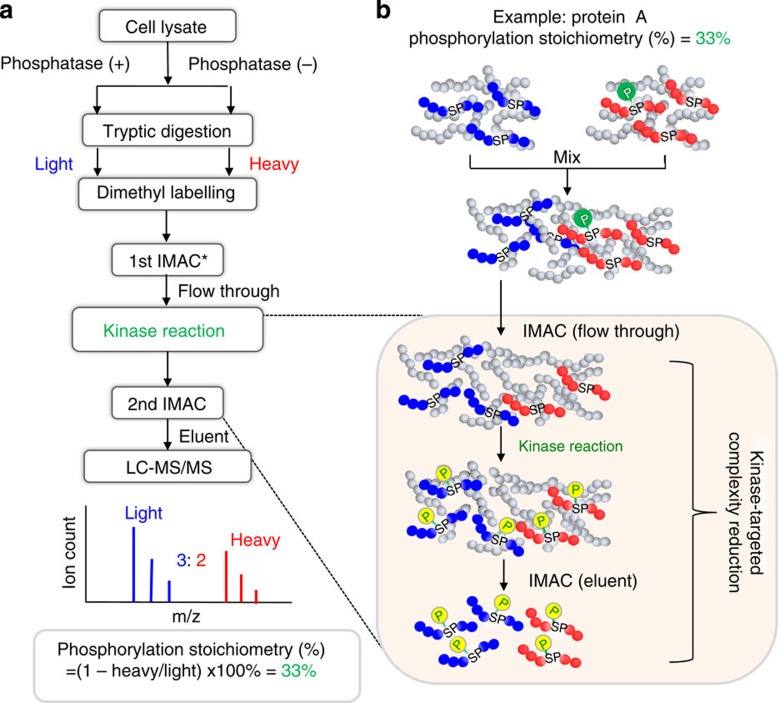
Figure 1. The basic principle of the motif-targeting quantitative proteomic approach.
(a) Two identical aliquots of tryptic peptides are either mock or phosphatase treated followed by isotopic tagging. Then, the mixing fraction is purified by IMAC. In the flow through of IMAC purification (b), the dephosphorylated peptides from phosphatase-treated aliquot (blue, light isotope labelled) will represent the total peptides, while the unphosphorylated counterparts in the untreated aliquot (red, heavy isotope labelled) will represent the fraction of initial unphosphorylated amount. The mixture of dephosphorylated and unphosphorylated peptides in the flow through is subjected to phosphorylation via a kinase reaction. The motif-targeting phosphopeptides are purified by IMAC. The ratio of heavy/light will represent the fraction of initially unphosphorylated amount and the phosphorylation stoichiometry can be calculated by the formula shown in a. *: In this IMAC step, the phosphopeptides identified from the IMAC eluent can be used to derive the phosphorylation sequence motif and potential kinase for subsequent kinase reaction.